Acids, Bases, and Salts: The Ultimate Guide for IGCSE & O Level Chemistry Mastery
Your complete resource for IGCSE Chemistry 0620, O Level Chemistry 5070, AQA, and Edexcel syllabuses. Packed with definitions, reactions, notes, and exam secrets.
Boost Your Study Mood!
Kickstart your revision with positive energy. A focused mind learns faster!
Part 1: Core Concepts & Definitions
Understanding the fundamental definitions is the first step to conquering this topic. We'll explore the key theories you need to know for your O Level Chemistry 5070 or IGCSE Chemistry 0620 exam[citation:1][citation:6].
1. The Arrhenius Theory (The Foundation)
Proposed by Svante Arrhenius in 1884, this is the simplest definition and the one most frequently tested[citation:1].
- Arrhenius Acid: A substance that increases the concentration of H⁺ ions (protons) when dissolved in water[citation:1]. Example: HCl → H⁺ + Cl⁻
- Arrhenius Base: A substance that increases the concentration of OH⁻ ions (hydroxide ions) when dissolved in water[citation:1]. Example: NaOH → Na⁺ + OH⁻
Key Fact: Free H⁺ ions don't really exist in water. They instantly bond with a water molecule to form the hydronium ion, H₃O⁺[citation:1]. So, HCl(aq) + H₂O(l) → H₃O⁺(aq) + Cl⁻(aq).
2. Physical Properties: How to Identify Them
You can often identify acids and bases by their observable properties[citation:4][citation:10].
| Property | Acids | Bases |
|---|---|---|
| Taste | Sour (e.g., lemon juice, vinegar)[citation:5] | Bitter (e.g., soap, baking soda)[citation:5] |
| Touch/Texture | Usually feel like water. | Feel soapy and slippery[citation:4]. |
| Effect on Litmus | Turn blue litmus paper red[citation:5]. | Turn red litmus paper blue[citation:5]. |
| pH Value | pH < 7[citation:4] | pH > 7[citation:4] |
| Reaction with Metals | React with many metals to produce a salt and hydrogen gas (H₂)[citation:2][citation:10]. | Typically do not react with most metals[citation:4]. |
3. What is a Salt?
A salt is an ionic compound formed when an acid reacts with a base, a process called neutralization[citation:5][citation:6].
General Equation: Acid + Base → Salt + Water[citation:2]
For example: Hydrochloric Acid + Sodium Hydroxide → Sodium Chloride + Water[citation:2]
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
Salts are made up of positive ions (cations, often from the base) and negative ions (anions, from the acid). Not all salts are neutral—their pH in water depends on the strength of the parent acid and base[citation:3][citation:9].
Part 2: Common Acids & Bases in Daily Life and the Lab
Chemistry isn't just in textbooks. Here are examples you encounter every day[citation:5][citation:8].
🛒 Everyday Acids
- Citric Acid: In lemons, oranges, and other citrus fruits.
- Acetic Acid (CH₃COOH): The main component of vinegar[citation:8].
- Lactic Acid: Found in yogurt and sour milk.
- Tartaric Acid: In tamarind and grapes.
- Carbonic Acid (H₂CO₃): In carbonated soft drinks.
- Hydrochloric Acid (HCl): A strong acid found in our stomach gastric juices[citation:8].
🧼 Everyday Bases
- Sodium Hydroxide (NaOH, Caustic Soda): In drain and oven cleaners[citation:8].
- Calcium Hydroxide (Ca(OH)₂, Slaked Lime): Used in mortar and whitewash[citation:8].
- Ammonia (NH₃): In many household glass and surface cleaners[citation:8].
- Magnesium Hydroxide (Mg(OH)₂): The active ingredient in "Milk of Magnesia," an antacid.
- Sodium Bicarbonate (NaHCO₃, Baking Soda): Used in baking and as a mild cleaning agent.
Part 3: Key Reactions You MUST Know (with Equations)
This is the heart of the exam. Memorize the patterns, not just individual equations[citation:2][citation:10].
🎯 Exam Tip:
For O Level Chemistry 5070 past papers and IGCSE Chemistry 0620 past papers, you are almost always required to write balanced chemical equations. Practice these general patterns!
1. Acid + Metal → Salt + Hydrogen Gas
A classic test for acids[citation:2][citation:10].
- Example: Zinc + Hydrochloric Acid → Zinc Chloride + Hydrogen[citation:10]
Zn(s) + 2HCl(aq) → ZnCl₂(aq) + H₂(g) - Why it matters: The "pop" test for hydrogen gas is a frequent O Level Chemistry 5070 ATP (Alternative to Practical) question.
2. Acid + Base (Neutralization) → Salt + Water
The most fundamental acid-base reaction[citation:2][citation:6].
- Example: Sulfuric Acid + Sodium Hydroxide → Sodium Sulfate + Water[citation:8]
H₂SO₄(aq) + 2NaOH(aq) → Na₂SO₄(aq) + 2H₂O(l) - Net Ionic Equation: The core of any strong acid-strong base neutralization is: H⁺(aq) + OH⁻(aq) → H₂O(l)[citation:2].
3. Acid + Metal Carbonate → Salt + Water + Carbon Dioxide
Another key test for acids[citation:4][citation:10].
- Example: Calcium Carbonate (Marble) + Hydrochloric Acid → Calcium Chloride + Water + CO₂
CaCO₃(s) + 2HCl(aq) → CaCl₂(aq) + H₂O(l) + CO₂(g) - Why it matters: The effervescence (fizzing) due to CO₂ is a standard identification test.
4. Acidic/Amphoteric Oxides & Basic Oxides
Oxides also show acid-base character[citation:10].
- Acidic Oxide + Base → Salt + Water: e.g., CO₂ + Ca(OH)₂ (limewater) → CaCO₃ + H₂O[citation:10].
- Basic Oxide + Acid → Salt + Water: e.g., CuO + H₂SO₄ → CuSO₄ + H₂O[citation:10].
- Amphoteric Oxides (e.g., Al₂O₃, ZnO) react with both acids and strong bases[citation:4].
Watch a Past Paper Solved by an Expert
See how a top teacher tackles an IGCSE Chemistry 0620 past paper question.
Part 4: pH, Salt Hydrolysis, and Predicting Salt Acidity
Why is NaCl neutral but NH₄Cl acidic? The answer lies in hydrolysis[citation:3].
The pH of Salts: The 4 Rules
When a salt dissolves, its ions can react with water. This is hydrolysis. Use this table to predict the pH of a salt solution[citation:3]:
| Salt Made From... | Example | pH of Solution | Reason (Hydrolysis) |
|---|---|---|---|
| Strong Acid + Strong Base | NaCl (HCl + NaOH) | = 7 (Neutral) | Neither ion reacts with water. Spectator ions only[citation:3]. |
| Strong Acid + Weak Base | NH₄Cl (HCl + NH₃) | < 7 (Acidic) | The cation (NH₄⁺) from the weak base donates H⁺ to water[citation:3]. |
| Weak Acid + Strong Base | CH₃COONa (CH₃COOH + NaOH) | > 7 (Basic/Alkaline) | The anion (CH₃COO⁻) from the weak acid accepts H⁺ from water, leaving OH⁻[citation:3]. |
| Weak Acid + Weak Base | CH₃COONH₄ | ≈ 7 (Near Neutral)* | Depends on relative strengths (Ka vs Kb). Usually close to 7[citation:3]. |
*Requires comparison of acid and base dissociation constants (Ka, Kb) for exact pH.
Struggling with the Whole Syllabus?
Our structured crash course covers ALL of O Level Chemistry 5070 and IGCSE Chemistry 0620 in record time.
🔥 Get 10% Off the Complete Chemistry Crash Course!Part 5: Exam Mastery & Your Free Resource Hub
Success comes from the right resources. Here is everything you need.
📝 Top 5 Exam Strategies for Acids, Bases, Salts
- Memorize the Reaction Patterns: Acid+Metal, Acid+Carbonate, Neutralization. Write them out daily.
- Know Your Tests: H₂ (pop test), CO₂ (limewater turns milky), NH₃ (turns damp red litmus blue).
- Predict Salt pH: Use the 4 rules from Part 4. This is a high-mark discriminator question.
- Practice Ionic Equations: Especially the net ionic equation for neutralization (H⁺ + OH⁻ → H₂O).
- Use the O Level Chemistry Formula Sheet: Know what's on it and how to use it fast.
📚 Your Ultimate Chemistry Download Hub
Download comprehensive, exam-focused notes for every major topic. Click the links to get the PDFs from Google Drive.
Pro Tip: Combine these with the Save My Exams Notes for IGCSE Chemistry 0620 for unbeatable revision.
Learn from the Best: Live Classes & Expert Guidance
Meet Your Guide: Prof. Faisal Janjowa
With over 15 years of experience specializing in Cambridge IGCSE Chemistry 0620 and O Level Chemistry 5070, Prof. Janjowa has helped thousands of students achieve A* and A grades. His teaching focuses on simplifying complex concepts and drilling exam technique.
Live Class Schedule: Weekly classes on Stoichiometry & Moles, Organic Chemistry, and Acids & Bases Revision. New batch starting soon. Check the updated syllabus & schedule here.
Watch a full O Level Paper 4 solved: See Prof. Janjowa's problem-solving approach in action.
Part 6: Last-Minute Revision & Cheat Sheets
Crunch time? These resources are your lifesaver.
- Last Minute Revision Notes for O Level Chemistry 5070
- O Level Chemistry Notes PDF (All Chapters)
- How to Calculate Empirical Formulas Easily (Related to salt formulas)
Ultimate Cation/Anion & Gas Test Cheat Sheet
Memorize this table for identification questions in Paper 4 (ATP).
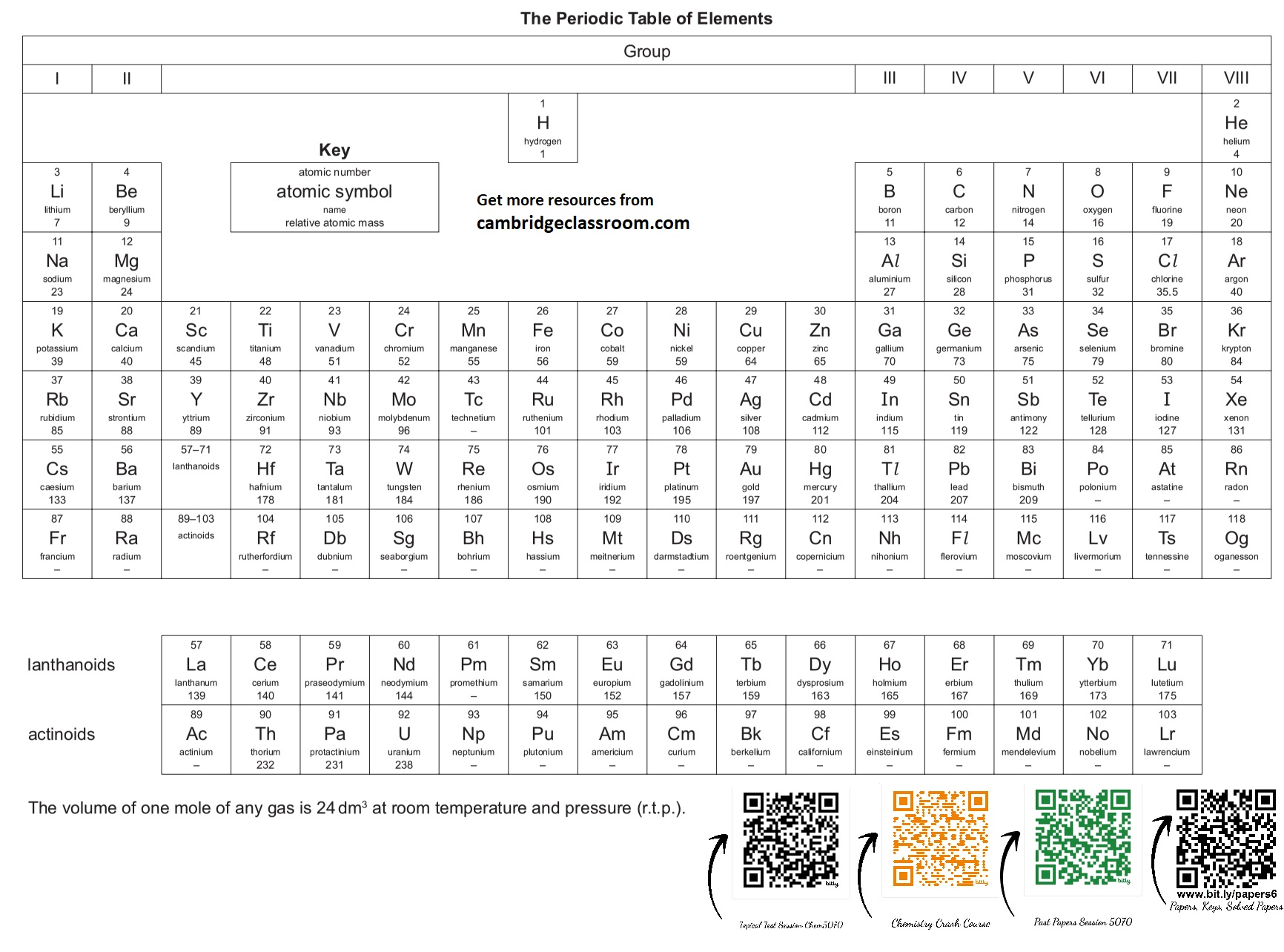
Learn the Periodic Table in a Song!
Knowing Group I & II metals (which react with acids) and Group VII (halogens, part of acids) is crucial. This makes it fun!
Frequently Asked Questions (FAQs)
Step 1: Litmus Test. Blue to Red = Acid. Red to Blue = Base. No change could be a neutral salt (but not always).
Step 2: Reaction Tests. If it's an acid, it will fizz with a carbonate or a reactive metal. A base will feel soapy.
Step 3: pH Measurement. Use universal indicator or a pH probe for a definitive number[citation:4][citation:5].
Neither. NaCl is a neutral salt. It is formed from a strong acid (HCl) and a strong base (NaOH). When dissolved, neither Na⁺ nor Cl⁻ ions react with water, so the pH remains 7[citation:3][citation:9].
Stomach pain can be from excess hydrochloric acid (HCl). Antacids like magnesium hydroxide (Mg(OH)₂) are bases. They undergo a neutralization reaction with the stomach acid: Mg(OH)₂ + 2HCl → MgCl₂ + 2H₂O. This reduces acidity and relieves pain[citation:8][citation:10].
This is a common confusion! Strength refers to how completely an acid dissociates into ions. A strong acid (like HCl) dissociates 100%. A weak acid (like CH₃COOH) does not. Concentration is about how much acid is dissolved in a given volume of water. You can have a dilute strong acid or a concentrated weak acid[citation:1][citation:10].
What Our Students Say
- sydney-sixers-vs-sydney-thunder
- jake-paul-vs-anthony-joshua
- epstein-files
- ecp
- raat-akeli-hai-the-bansal-murders
- daniel-sams
- imran-khan
- xiaomi-redmi-note-15
- sam-konstas
- indian-videos
- dv-vs-sw
- gta-vice-city-browser
- aleem-khan-palwasha-khan
- ilia-topuria
- andrew-tate-vs-chase-demoor
- oppo-pad-air-5
- chelsea
- sharjah-warriors-vs-desert-vipers
- shah-mahmood-qureshi
- osman-hadi
- the-great-flood-netflix
- justice-tariq-mehmood-jahangiri
- t20-world-cup-2026
- greg-biffle
- javed-akhtar
- sreenivasan
- anthony-joshua
- newcastle-vs-chelsea
- andrew-tate-vs-chase-demoor
- سمية-الألفي
- super-flu
- global-village
- t20-world-cup-2026
- mitsubishi-pajero
- marty-supreme
- سمية-الألفي
- ابو-بشت
- تشيلسي
- شهر-رجب
- chelsea
- sreenivasan
- epstein-files
- فيديو-هيفاء-وهبي
- anthony-joshua
- جبل-قاسيون
- الدوري-الفرنسي
- riyadh-airport-flight-delays
- حنان-ترك
- cricket-u19-asia-cup
- والدة-شيماء-جمال
- امم-افريقيا-2025
- ياسمين-عز-محمد-صبحي
- who-won-the-jake-paul-fight
- oklahoma-football
- bill-clinton
- lamarr-wilson
- elise-stefanik
- andrew-tate
- cfp-schedule
- tyron-woodley
- bowen-yang
- texas-a&m-football
- thunder-vs-timberwolves
- newcastle-vs-chelsea
- chris-tucker
- gisele-bündchen
- timothee-chalamet
- dave-chappelle
- john-mateer
- jenna-marbles
- ryan-williams
- mike-tyson
- kay-adams
- diana-ross
- tyler-skaggs
- pam-bondi
- ole-miss-football
- höfner-bass-paul-mccartney
- nazan-eckes
- lets-dance-weihnachtsshow-live
- bill-clinton
- boxkampf-anthony-joshua-jake-paul
- fck-–-1.-fc-magdeburg
- jeffrey-epstein
- dom-taylor
- dortmund-gladbach
- paul-vs-joshua
- ghislaine-maxwell
- aktie
- andrew-tate-vs-chase-demoor
- fortuna-düsseldorf
- ksc
- rot-weiss-essen
- newcastle
- hsv-frankfurt
- hansa-rostock
- nick-woltemade
- le-grand-bornand
- sophie-marceau
- disney+
- vfl-osnabrück
- wwe-friday-night-smackdown-ergebnisse
- acids-bases-salts
- acid-bases-salts-in-hindi
- acid-base-salt-in-hindi-meaning
- acid-base-salt-in-chemistry
- acid-base-salt-for-class-7
- acids-bases-in-salts
- acids-bases-and-salts-for-class-10
- acids-bases-and-salts-in-telugu
- acids-bases-and-salts-for-class-10-notes
- acids-bases-and-salts-for-class-10-pdf
- acids-bases-and-salts-for-class-10-mcq
- how-to-differentiate-between-acid-base-and-salt
- acid-base-salt-with-example
- acids-bases-and-salts-by-prashant-kirad
- acids-bases-and-salts-in-class-7
- acids-bases-and-salts-in-science
- acid-bases-and-salt-of-class-10th
- acids-bases-and-salts-in-hindi-meaning
- acids-bases-and-salts-in-chemistry
- acids-bases-and-salts-in-kannada
- acids-bases-and-salts-in-kannada-meaning
- acids-bases-and-salts-for-class-7
- acids-bases-and-salts-for-jss3
- acids-bases-and-salts-for-grade-7
- acids-bases-and-salts-for-class-7-pdf
- acids-bases-and-salts-for-class-7th
- acids-bases-and-salts-for-class-10-icse
- acid-base-salt-in-tamil
- acid-base-salt-in-bengali
- acid-base-salt-in-our-daily-life
- acids-bases-and-salts-in-daily-life
- acid-base-to-salt-water
- acids-bases-and-salts-past-papers
- acid-base-salt-plus-water
- acids-plus-bases
- acids-bases-and-salts-pre-lab
- acid-base-and-salt-in-hindi-class-7
- how-to-identify-salts-bases-and-acids
- acids-bases-and-salts-dissociate-into-and
- acids-bases-salts
- what-are-acids-bases-salts
- what-are-acids-bases-and-salts-worksheet
- what-are-the-uses-of-acids-bases-and-salts
- how-are-salts-related-to-acids-and-bases
- what-are-acids-bases-and-salts-with-examples
- what-are-the-characteristics-of-acids-bases-and-salts
- what-are-the-properties-of-acids-bases-and-salts
- what-are-acids-bases-and-salts-in-chemistry
- are-acids-bases-and-salts-electrolytes
- what-are-acids-bases-and-salts-class-10
- are-salts-acids-or-bases
- can-bases-be-salts
- can-salts-be-acidic
- can-salts-produce-acids-and-bases
- can-acids-and-bases-mix
- can-acids-be-bases
- are-acids-salts
- do-all-bases-form-salts-with-acids
- do-acids-bases-and-salts-conduct-electricity
- do-acids-and-bases-form-salts
- how-do-the-properties-of-acids-bases-and-salts-compare
- what-do-acids-bases-and-salts-have-in-common
- do-acids-react-with-bases-to-form-salts
- what-do-you-mean-by-acids-bases-and-salts
- how-do-acids-bases-and-salts-compare
- does-acid-dissolve-salt
- what-property-do-acids-bases-and-salts-have-in-common
- what-are-the-acids-bases-and-salts
- how-to-identify-acids-bases-and-salts
- how-to-differentiate-between-acids-bases-and-salts
- how-to-classify-compounds-as-acids-bases-or-salts
- how-are-salts-different-from-acids-and-bases
- how-acids-and-bases-work
- how-are-salts-and-acids-similar
- how-do-acids-bases-and-salts-affect-your-daily-life
- how-do-acids-and-bases-form-salts-through-chemical-reactions
- what-is-acids-bases-salts
- what-is-the-difference-between-acids-bases-and-salts
- what-is-the-meaning-of-acids-bases-and-salts
- what-is-acids-bases-and-salts-class-10
- what-is-acids-bases-and-salts-in-chemistry
- what-is-acids-bases-and-salts-class-7
- what-is-the-hindi-meaning-of-acids-bases-and-salts
- how-should-acids-and-bases-be-stored
- why-should-acids-and-bases-not-be-stored-together
- what-are-acids-salts-and-bases
- what-are-acids-bases-and-salts
- when-acids-and-bases-are-mixed
- when-acids-are-added-to-a-solution-the-ph-should
- when-acids-and-bases-react-what-are-the-products
- where-are-acids-and-bases-found
- where-are-acids-and-bases-located
- where-are-acids-and-bases-commonly-used
- where-can-you-find-acids-and-bases-in-everyday-life
- where-are-acids-and-bases-found-on-the-ph-scale
- which-salts-are-acidic
- which-salts-are-basic
- which-salts-produce-acidic-solutions
- which-salts-form-basic-solutions
- which-salts-produce-basic-solutions
- why-do-acids-bases-and-salts-conduct-electricity
- why-are-acids-bases-and-salts-electrolytes
- why-are-bases-and-acids-important
- why-are-acids-and-bases-electrolytes
- why-are-acids-and-bases-important-in-biology
- why-are-acids-and-bases-important-in-chemistry
- will-acids-and-bases-neutralize-each-other
- will-salt-neutralize-acid
- what-are-acids-bases-and-salts-worksheet-answers
- acids-bases-salts
- class-10-science-acids-bases-salts
- acids-bases-salts-and-buffers
- acids-bases-salts-class-10
- acids-bases-salts-class-10-notes
- acids-bases-salts-class-7
- acids-bases-salts-class-10-pdf
- acids-bases-salts-notes
- acids-bases-salts-pdf
- acids-bases-salts-class-10-extra-questions
- acids-bases-salts-pyqs
- acids-bases-salts-and-oxides
- acids-bases-salts-activities
- acid-base-salt-and-water
- acid-base-salt-and
- acid-base-salt-all-formula
- acid-base-salt-and-indicator
- acid-base-salt-assignment
- acids-bases-and-salts-class-10
- acids-bases-and-salts-class-10-notes
- acids-bases-and-salts-class-7
- acids-bases-and-salts-class-7-questions-and-answers-pdf
- acids-bases-and-salts-notes-pdf
- acids-bases-and-salts-class-10-questions-and-answers-pdf
- acids-bases-and-salts-class-10-pdf
- acids-bases-and-salts-class-7-pdf
- acids-bases-and-salts-class-10-notes-pdf-download
- acids-bases-and-salts-notes-pdf-download
- acids-bases-and-salts-by-prashant-kirad
- acid-bases-and-salts-bank-of-biology
- acid-base-and-salt-basic-science-jss3
- acid-base-and-salt-baking-soda
- acid-base-or-salt-ba-oh-2
- acid-base-salt-difference-between
- acids-bases-and-salts-case-based-questions-class-10
- acids-bases-and-salts-notes-by-prashant-kirad
- acids-bases-and-salts-case-based-questions-class-7
- competency-based-questions-on-acids-bases-and-salts-class-10
- bank-of-biology-class-10-acids-bases-and-salts
- case-based-questions-on-acids-bases-and-salts-class-10
- case-based-questions-on-acids-bases-and-salts-class-7
- ncert-science-book-class-10-acids-bases-and-salts
- ncert-science-book-class-7-acids-bases-and-salts
- differentiate-between-acids-bases-and-salts
- acids-bases-salts-class-7-extra-questions
- acids-bases-salts-class-10-ncert-solutions
- acids-bases-salts-class-10-icse
- acids-bases-salts-class-7-notes
- acids-bases-salts-class-8
- class-10-acids-bases-salts-notes
- class-10-acids-bases-salts
- class-10-acids-bases-salts-pdf
- class-10-acids-bases-salts-mcq
- class-10-acids-bases-salts-solutions
- class-7-acids-bases-salts
- chemistry-acids-bases-salts
- class-10-acids-bases-salts-ncert-solutions
- acids-bases-salts-definition
- acids-bases-salts-diagram
- acid-base-salt-difference
- acid-base-salt-drawing
- acid-base-salt-dash
- acid-base-salt-define
- acid-base-salt-definition-class-10
- acid-base-salt-definition-with-example
- acids-bases-and-salts-diagram-class-7
- acids-bases-and-salts-drawing-easy
- difference-between-acids-bases-salts
- define-acids-bases-salts
- class-7-acids-bases-and-salts-notes-pdf-download
- acids-bases-and-salts-class-10-pdf-download
- acids-bases-and-salts-class-10-ppt-free-download
- acids-bases-and-salts-definition
- acids-bases-and-salts-class-7-ppt-free-download
- acids-bases-and-salts-notes-pdf-free-download
- acids-bases-salts-exemplar
- acids-bases-salts-extra-questions
- acids-bases-salts-examples
- acid-bases-salts-exercise
- acid-base-salt-extra-questions-class-10
- acid-base-salt-extra-questions-class-7
- acid-base-salt-experiment
- acid-base-salt-explanation
- acid-base-salt-equation
- acid-base-salt-extra-question-answer
- examples-of-acids-bases-salts
- acids-bases-and-salts-class-7-extra-questions-and-answers
- class-7-acids-bases-and-salts-extra-questions
- acids-bases-and-salts-class-10-extra-questions
- acids-bases-and-salts-extra-questions
- class-7-science-acids-bases-and-salts-extra-questions
- ncert-exemplar-class-10-acids-bases-and-salts-solutions
- ncert-exemplar-class-7-science-acids-bases-and-salts
- acids-bases-and-salts-class-10-exercise
- ncert-exemplar-class-10-acids-bases-and-salts
- acid-base-salt-formula
- acid-base-salt-form-4
- acid-base-salt-flow-chart
- acid-base-and-salts-file-type-ppt
- acid-base-salt-formation
- acid-base-salt-for-class-7
- acid-base-form-salt
- acids-bases-and-salts-for-class-10
- acids-bases-and-salts-form-4-notes
- acids-bases-and-salts-for-class-10-notes
- questions-on-acids-bases-and-salts-for-class-7
- important-questions-from-acids-bases-and-salts
- notes-for-acids-bases-and-salts
- important-questions-from-acids-bases-and-salts-class-10
- ncert-solutions-for-class-10-acids-bases-and-salts
- mcq-from-acids-bases-and-salts-class-10
- notes-for-acids-bases-and-salts-class-10
- notes-for-class-10-science-acids-bases-and-salts
- acids-bases-salts-grade-7
- acids-bases-salts-grade-10
- acid-base-gives-salt-water-example
- acid-base-gives-salt
- acid-base-gives-salt-water-reaction
- acid-base-gives-salt-water-this-reaction-is-known-as
- acid-base-gives-salt-hydrogen
- acids-bases-and-salts-gcse
- acids-bases-and-salts-grade-7-extra-questions
- acids-bases-and-salts-gcse-questions
- acids-bases-and-salts-questions-and-answers-pdf-gcse
- grade-7-acids-bases-and-salts
- give-a-few-uses-of-acids-bases-and-salts-respectively
- acids-bases-and-salts-class-10-ppt-google-docs
- grade-10-acids-bases-and-salts-notes
- grade-7-acids-bases-and-salts-assertion-reason-questions
- grade-7-acids-bases-and-salts-extra-questions
- lesson-plan-on-acids-bases-and-salts-for-grade-7
- grade-10-acids-bases-and-salts-pdf
- acid-base-salt-hindi
- acid-base-salt-hindi-meaning
- acid-base-salt-hydrogen
- acid-base-salt-h2o
- acid-base-and-salts-handwritten-notes
- acid-base-salt-hydrogen-gas
- acid-base-salt-hindi-notes
- acid-base-salt-hard-questions
- acid-base-salt-h2
- acid+base=-salt-h20
- acids-bases-and-salts-meaning-in-hindi
- acids-bases-and-salts-in-hindi
- class-10-acids-bases-and-salts-handwritten-notes
- hots-questions-on-acids-bases-and-salts-class-7
- hots-questions-on-acids-bases-and-salts-class-10
- acids-bases-and-salts-handwritten-notes
- acids-bases-and-salts-class-10-pdf-notes-handwritten
- class-10-acids-bases-and-salts-notes-in-hindi
- hots-questions-on-acids-bases-and-salts
- acids-bases-salts-important-questions
- acids-bases-salts-important-questions-class-10
- acids-bases-salts-igcse
- acid-bases-salts-intext-questions
- acid-bases-salts-in-hindi
- acid-base-salt-introduction
- acid-base-salt-indicator
- acid-base-salt-in-hindi-meaning
- acid-base-and-salts-class-10-icse
- acid-base-salt-in-chemistry
- igcse-chemistry-acids-bases-salts
- acids-bases-and-salts-important-questions
- acids-bases-and-salts-class-10-icse
- acids-bases-and-salts-class-10-intext-questions
- acids-bases-and-salts-class-10-icse-notes
- acids-bases-and-salts-intext-questions
- acids-bases-and-salts-igcse
- acids-bases-and-salts-is-chemistry-or-physics
- acids-bases-and-salts-jss3
- acids-bases-and-salts-jss3-basic-science
- acids-bases-and-salts-jss3-notes
- acids-bases-and-salts-jee
- acid-base-and-salt-jss3-lesson-note
- acid-base-and-salt-jss3-basic-science-notes
- acid-base-and-salt-jss3-basic-science-pdf
- acid-base-and-salt-jamb
- acid-base-and-salt-jss3-lesson-note-pdf
- acid-base-and-salt-js3
- acids-bases-and-salts-class-7-jkbose
- acids-bases-and-salts-class-7-questions-and-answers-jkbose
- acids-bases-and-salts-class-7-jkbose-solutions
- acids-bases-and-salts-iit-jee-pdf
- jamb-questions-on-acids-bases-and-salts
- acids-bases-and-salts-iit-jee-questions
- acids-bases-and-salts-class-10-notes-jagran-josh
- acids-bases-and-salts-class-10-jkbose
- acid-base-salt-ka-hindi
- acid-base-salt-kya-hai
- acid-base-salt-ki-definition
- acid-base-salt-ka-diagram
- acid-base-and-salts-ka-notes
- acid-base-salt-ka-example
- acid-base-salt-ka-formula
- acid-base-salt-ka-hindi-meaning
- acid-base-salt-ka
- acids-bases-and-salts-key-points
- acids-bases-and-salts-class-10-notes-prashant-kirad
- kseeb-solutions-for-class-7-science-acids-bases-and-salts
- prashant-kirad-acids-bases-and-salts-notes
- kseeb-solutions-for-class-10-science-acids-bases-and-salts
- acids-bases-and-salts-class-10-prashant-kirad
- prashant-kirad-acids-bases-and-salts
- kseeb-solutions-for-class-10-acids-bases-and-salts
- acids-bases-and-salts-class-10-key-points
- acids-bases-and-salts-class-9-samacheer-kalvi
- acid-base-salt-litmus-paper
- acid-base-and-salts-lesson-plan
- acid-base-and-salts-long-question-answer
- acid-base-and-salts-lesson
- acid-base-salt-list
- acids-bases-and-salts-lesson-plan-class-10
- acids-bases-and-salts-lesson-pdf
- acids-bases-and-salts-lesson-explanation
- acids-bases-and-salts-learn-cbse
- acids-bases-and-salts-long-questions
- lab-14-acids-bases-salts-and-buffers
- 10th-class-acids-bases-and-salts-lesson
- lesson-plan-for-acids-bases-and-salts-class-10
- learn-cbse-class-7-science-acids-bases-and-salts
- learn-cbse-class-10-acids-bases-and-salts
- acids-bases-and-salts-class-7-mcq-learn-insta
- learning-outcomes-of-acids-bases-and-salts-class-7
- uses-of-acids-bases-and-salts-in-our-daily-life
- acids-bases-salts-mcq
- acids-bases-salts-mind-map
- acids-bases-salts-mcq-class-7
- acids-bases-salts-mcq-test
- acids-bases-salts-meaning-in-hindi
- acid-bases-salts-mcq-class-10
- acid-bases-salts-mcq-class-10-icse
- acid-bases-salts-mcq-class-10-cbse
- acid-bases-salts-mcq-questions
- acid-base-salts-mcq-class-11
- mcq-acids-bases-salts-class-10
- mcq-on-acids-bases-salts-class-7
- mcq-on-acids-bases-and-salts-class-10-pdf
- multiple-choice-questions-on-acids-bases-and-salts-pdf
- mcq-on-acids-bases-and-salts-class-7-pdf
- acids-bases-and-salts-class-7-mcq-online-test
- acids-bases-and-salts-mcq
- acids-bases-and-salts-class-10-mcq-online-test
- mcq-questions-on-acids-bases-and-salts-class-10
- acids-bases-salts-ncert
- acids-bases-salts-ncert-solutions
- acids-bases-salts-notes-pdf
- acids-bases-salts-notes-class-10
- acids-bases-salts-ncert-pdf
- acids-bases-salts-ncert-exemplar
- acid-bases-salts-ncert-class-10
- acid-base-salts-ncert-question-answer
- acid-base-salt-notes-class-10-pdf
- notes-of-chemistry-class-10-acids-bases-salts
- ncert-acids-bases-salts
- ncert-solutions-class-10-acids-bases-salts
- ncert-class-10-acids-bases-salts-pdf
- ncert-class-10-acids-bases-salts
- acids-bases-and-salts-notes
- acids-bases-and-salts-class-7-notes
- acids-bases-salts-oxides
- acid-base-salt-online-test
- acid-base-salt-one-shot
- acid-base-salt-objective-questions
- acids-bases-or-salts
- acid-base-or-salt-worksheet
- acid-base-or-salt-koh
- acid-base-or-salt-naoh
- acid-base-or-salt-hno3
- acid-base-or-salt-in-hindi
- are-salts-acids-or-bases
- notes-of-acids-bases-and-salts
- notes-on-acids-bases-and-salts-class-7
- ncert-solutions-of-acids-bases-and-salts-class-10
- questions-on-acids-bases-and-salts-class-10-with-answers
- questions-and-answers-on-acids-bases-and-salts-class-7
- notes-on-acids-bases-and-salts-pdf
- acids-bases-salts-pdf-class-10
- acids-bases-salts-pmt
- acids-bases-salts-ppt
- acids-bases-salts-ppt-class-7
- acids-bases-salts-pdf-ncert
- acid-bases-salts-ppt-class-10
- acid-bases-salts-previous-year-question
- picture-of-acid-bases-and-salts
- properties-of-acids-bases-salts
- acids-bases-and-salts-class-10-ncert-pdf
- acids-bases-salts-question-answers
- acids-bases-salts-questions
- acids-bases-salts-quiz
- acids-bases-salts-quiz-questions
- acid-bases-salts-questions-class-10
- acid-base-salt-question-answer-class-10
- acid-base-salt-question-answer-class-7
- acid-base-salt-question-answer-class-10th
- acid-base-and-salts-question-paper
- acid-base-salt-question-pdf
- acids-bases-and-salts-class-10-questions-and-answers
- acids-bases-and-salts-questions-and-answers-pdf
- acids-bases-and-salts-class-7-questions-and-answers
- acid-bases-salts-reactions
- acid-base-salt-related-questions
- acid-base-salt-rules
- acid-base-reaction-salt-and-water
- acids-bases-and-salts-revision-notes
- acids-bases-and-salts-revision
- acids-bases-and-salts-revision-questions
- acids-bases-and-salts-running-notes
- acids-bases-and-salts-rapid-revision
- acids-bases-and-salts-report-sheet
- assertion-reason-questions-on-acids-bases-and-salts-class-7
- all-reactions-of-acids-bases-and-salts-class-10
- acids-bases-and-salts-class-10-notes-study-rankers
- acids-bases-and-salts-class-7-assertion-and-reason
- acids-bases-and-salts-class-10-assertion-and-reason
- assertion-reason-questions-on-acids-bases-and-salts-class-10
- acids-bases-and-salts-class-10-study-rankers
- acids-bases-salts-solutions
- acid-base-salt-solution-class-10
- acid-base-and-salts-sample-paper
- acid-base-and-salts-short-notes
- acid-base-and-salts-summary
- acids-bases-and-salts-short-notes-class-10
- acids-bases-and-salts-summary-class-10
- acids-bases-and-salts-solutions-class-7
- acids-bases-and-salts-science
- class-7-science-chapter-4-acids-bases-and-salts
- acids-bases-and-salts-class-10-solutions
- class-7-science-acids-bases-and-salts
- ncert-solutions-acids-bases-and-salts-class-7
- class-7-science-chapter-5-acids-bases-and-salts
- class-7-science-chapter-acids-bases-and-salts
- case-study-questions-on-acids-bases-and-salts-class-7
- acids-bases-and-salts-ncert-solutions
- class-10-science-ch-acids-bases-and-salts
- class-10-science-chapter-acids-bases-and-salts-notes
- acids-bases-salts-test
- acid-bases-salts-test-paper
- acid-base-salts-theory
- acid-base-salt-table
- acid-bases-salts-tnpsc-questions
- acid-bases-salts-tnpsc-notes
- acid-bases-salts-tnpsc-questions-in-tamil
- acid-base-salt-tlm
- acid-base-and-salts-topic
- class-10-acid-base-and-salts-test
- the-four-classifications-of-compounds-are-acids-bases-salts-and
- acids-bases-and-salts-class-10-test-paper-pdf
- test-on-acids-bases-and-salts-class-7
- important-topics-in-acids-bases-and-salts-class-10
- acids-bases-and-salts-class-10-textbook-pdf
- acid-base-salt-upsc
- acid-base-salt-uses
- acids-bases-and-salts-used-in-our-daily-life
- acids-bases-and-salts-unacademy
- acids-bases-and-salts-used-in-therapeutic-processes
- acid-base-salt-water-uses
- acid-base-and-salt-upsc-notes
- acids-bases-and-salts-in-urdu
- acids-bases-and-salts-their-uses-in-daily-life
- acids-bases-and-salts-meaning-in-urdu
- uses-of-acids-bases-salts
- write-any-three-uses-each-of-acids-bases-and-salts
- uses-of-acids-bases-and-salts-class-7
- acids-bases-and-salts-upsc
- acid-base-salt-video
- acids-bases-and-salts-videos-for-class-7
- acids-bases-and-salts-vedantu
- acid-bases-and-salts-vaga-study
- acid-base-and-salts-very-short-questions
- acid-base-and-salts-very-short-question-answer
- acid-base-salt-ph-value
- acid-base-or-salt-vinegar
- acids-and-bases-vs-salts
- acids-bases-and-salts-class-10-videos-in-english
- vedantu-acids-bases-and-salts
- acids-bases-and-salts-class-10-animated-videos
- video-on-acids-bases-and-salts
- acids-bases-and-salts-class-10-notes-vedantu
- vedantu-class-10-chemistry-acids-bases-and-salts
- ph-value-of-acids-bases-and-salts
- acids-bases-and-salts-class-10-video
- acids-bases-salts-worksheet
- acids-bases-salts-worksheet-pdf
- acid-bases-salts-worksheet-class-7
- acid-bases-salts-worksheet-class-10
- acid-base-salt-water
- acid-base-salt-water-examples
- acid-base-salt-water-reaction
- acid-base-salt-water-equation
- acid-base-salt-water-heat
- acid-base-salt-water-what-type-of-reaction-is-this
- what’s-in-the-beaker-acids-bases-salts-and-buffers
- what-are-acids-bases-salts
- what’s-in-the-beaker-acids-bases-salts-and-buffers-answers
- write-uses-of-acids-bases-salts
- acids-bases-and-salts-worksheet-with-answers-pdf
- worksheet-on-acids-bases-and-salts-for-class-7
- igcse-acids-bases-and-salts-worksheet-with-answers-pdf
- acids-bases-and-salts-worksheet
- acids-bases-and-salts-class-7-worksheet-with-answers-pdf
- questions-on-acids-bases-and-salts-for-class-7-worksheet
- acids-bases-and-salts-xylem
- acids-bases-and-salts-class-x
- acids-bases-and-salts-class-10-xylem
- what-are-the-acids-bases-and-salts
- acids-bases-and-salts-explained
- acids-bases-salts-worksheet-answers
- acids-bases-and-salts-chemistry
- class-x-acids-bases-and-salts-notes
- class-x-acids-bases-and-salts
- class-x-acids-bases-and-salts-mcq
- class-x-science-acids-bases-and-salts
- class-x-acids-bases-and-salts-pdf
- class-x-acids-bases-and-salts-worksheet
- class-x-science-acids-bases-and-salts-notes
- class-x-acids-bases-and-salts-pyq
- acid-base-yields-salt+water
- acids-bases-and-salts-youtube
- acid-base-salt-previous-year-question-class-10
- acids-bases-and-salts-previous-year-board-questions
- acids-bases-and-salts-class-10-previous-year-questions
- acids-bases-and-salts-previous-year-questions
- grade-10-acids-bases-and-salts-previous-year-questions
- what-do-you-mean-by-acids-bases-and-salts
- salts-acids-and-bases-examples
- salts-acids-and-bases-chemistry
- salts-acids-and-bases-are-called
- znotes-chemistry-acids-bases-and-salts
- zinc-salts-examples
- zinc-salts-uses
- acid-bases-and-salts/0620
- acids-bases-and-salts-o-level-notes
- acids-bases-and-salts-o-level-questions
- acids-bases-and-salts-o-level-past-papers
- acids-bases-and-salts-o-level
- acids-bases-and-salts-o-level-chemistry
- acids-bases-and-salts-o-level-notes-pdf
- acids-bases-and-salts-#1
- 0620-acids-bases-and-salts
- 0-acids
- salts-acidic-basic-or-neutral
- acid-base-salt-10th-class
- acid-base-and-salts-10
- acids-bases-and-salts-1-mark-questions
- acids-bases-salts-class-10-mcq
- acids-bases-salts-class-10-solutions
- acids-bases-and-salts-class-10-important-questions
- acids-bases-and-salts-class-10-ppt
- acids-bases-and-salts-class-10-notes-pdf
- ch-acids-bases-and-salts-class-10
- acids-bases-and-salts-2-marks-questions
- acids-bases-and-salts-2-chemsheets-answers
- acids-bases-and-salts-2
- acid-base-salt-chapter-2
- acids-bases-and-salts-activity-2.1
- acids-bases-and-salts-activity-2.2
- acids-bases-and-salts-chapter-2-class-10
- acids-bases-and-salts-activity-2.3
- acids-bases-and-salts-activity-2.8
- acids-bases-and-salts-activity-2.9
- class-10-acids-bases-and-salts-activity-2.3
- chapter-2-acids-bases-and-salts
- chapter-2-acids-bases-and-salts-notes
- ch-2-acids-bases-and-salts
- class-10-chapter-2-acids-bases-and-salts-notes
- ch-2-acids-bases-and-salts-notes-class-10
- class-10-science-chapter-2-acids-bases-and-salts
- chapter-2-acids-bases-and-salts-question-answer
- ch-2-acids-bases-and-salts-intext-questions
- chemistry-chapter-2-acids-bases-and-salts-notes
- acids-bases-and-salts-3-marks-questions
- acid-base-and-salt-3d-model
- acids-bases-and-salts-class-10-3-mark-questions
- 3-acids-bases-and-salts
- what-are-acids-bases-and-salts-worksheet
- acids-bases-and-salts-section-3-salts
- section-3-acids-bases-and-salts-answers
- 10th-class-physics-3rd-lesson-acids-bases-and-salts
- unit-test-paper-3a-acids-bases-and-salts
- chapter-3-acids-bases-and-salts
- chapter-3-acids-bases-and-salts-notes
- chapter-3-acids-bases-and-salts-class-10-icse
- 3-mark-questions-on-acids-bases-and-salts-class-10
- class-7-science-chapter-3-acids-bases-and-salts
- chemistry-class-10-chapter-3-acids-bases-and-salts
- acids-bases-and-salts-chapter-4-class-7
- acids-bases-and-salts-table-4.1
- acids-bases-and-salts-chapter-4
- acids-bases-and-salts-table-4.2
- acids-bases-and-salts-table-4.3
- acids-bases-and-salts-table-4.4
- acids-bases-and-salts-table-4.5
- 4-acids-bases-and-salts
- 7th-class-science-4th-lesson-acids-bases-and-salts
- chapter-4-acids-bases-and-salts
- class-7-science-ch-4-acids-bases-and-salts-notes
- ch-4-acids-bases-and-salts-class-7
- chapter-4-acids-bases-and-salts-question-answer
- chapter-4-acids-bases-and-salts-notes
- acids-bases-and-salts-5-mark-questions
- acids-bases-and-salts-5070
- acids-bases-and-salts-chapter-5-class-7
- acids-bases-and-salts-chapter-5
- acids-bases-and-salts-class-10-5-mark-questions
- 5-acids-bases-and-salts
- 5-acids-bases-and-salts-exercise
- 5-acids-bases-and-salts-class-9
- 5-acids-bases-and-salts-exercise-class-9
- 5-acids-bases-and-salts-notes
- chapter-5-acids-bases-and-salts-question-answer
- class-7-science-ch-5-acids-bases-and-salts
- science-chapter-5-acids-bases-and-salts
- chapter-5-acids-bases-and-salts
- 5-mark-questions-from-acids-bases-and-salts
- acids-bases-and-salts-class-6
- acids-bases-and-salts-class-6-notes
- acids-bases-and-salts-grade-6
- acids-and-bases-6th-grade
- acids-and-bases-6th-grade-science
- experiment-6-acids-bases-and-salts
- class-7-science-chapter-6-acids-bases-and-salts
- experiment-6-acids-bases-and-salts-prelab
- experiment-6-report-sheet-acids-bases-and-salts
- experiment-6-prelaboratory-assignment-acids-bases-and-salts
- experiment-6-acids-bases-and-salts-lab-report
- acid-base-salt-7th-class
- acids-bases-and-salts-7th-class-notes
- acids-bases-and-salts-7th
- acids-bases-and-salts-7
- acids-bases-salts-class-7-worksheet
- acids-bases-salts-class-7-mcq
- acids-bases-salts-class-7-questions
- acids-bases-and-salts-class-8-pdf
- acids-bases-and-salts-class-8-notes
- acids-bases-and-salts-class-8-mcq
- acids-bases-and-salts-grade-8
- acids-bases-and-salts-class-8-pdf-notes
- acids-bases-and-salts-class-8-worksheet
- acids-bases-and-salts-class-8-pdf-solutions
- how-to-identify-salts-bases-and-acids
- acids-bases-and-salts-class-8
- class-8-chemistry-acids-bases-and-salts
- questions-on-acids-bases-and-salts-for-class-8
- acids-bases-and-salts-9th-class
- acids-bases-and-salts-9th
- acids-bases-and-salts-9
- acid-base-and-salts-class-9th-exercise
- acid-base-and-salt-9th-standard
- acids-bases-and-salts-class-9-questions-and-answers-pdf
- acids-bases-and-salts-class-9-notes-pdf
- acids-bases-and-salts-class-9-pdf
- acids-bases-and-salts-class-9-questions-and-answers
- 9th-class-science-acids-bases-and-salts-question-answer
- 9th-standard-science-acids-bases-and-salts
- 5-acids-bases-and-salts-exercise-9th
- 9th-science-acids-bases-and-salts-book-back-answers
- 9th-science-guide-acids-bases-and-salts
- class-9th-science-chapter-5-acids-bases-and-salts
- acids-bases-salts
- acids-bases-salts-pdf
- acids-bases-salts-class-10
- acids-bases-salts-notes
- acids-bases-salts-class-7
- acids-bases-salts-questions
- acids-bases-salts-class-10-notes
- acids-bases-salts-class-10-pdf
- acids-bases-salts-class-10-icse
- acids-bases-salts-class-7-questions
- acids-bases-salts-ncert-solutions-class-10
- acids-bases-&-salts
- acids-salts-and-bases
- chemistry-acids-bases-and-salts
- ch-2-acids-bases-and-salts
- science-acids-bases-and-salts
- acid-bases-salts-exemplar
- acids-bases-salts-class-10-extra-questions
- acids-bases-and-salts-solutions
- chemistry-acid-bases-salts
- g-class-10-notes-on-acids-bases-and-salts
- gcse-chemistry-acids-bases-and-salts
- acids-bases-and-salts-class-10
- acids-bases-and-salts
- acids-bases-and-salts-class-10-pdf
- acids-bases-and-salts-class-7
- acids-bases-and-salts-class-10-notes
- salts-acids-and-bases
- acids-bases-and-salts-notes-byjus
- kcl-acid-base-or-salt
- acid-bases-salts-mcqs
- m-class-10-notes-on-acids-bases-and-salts
- acid-bases-salts-ncert
- acids-bases-n-salts
- acids-and-bases-salts
- acids-bases-and-salts-notes-pdf
- acid-bases-salts-ppt
- acids-bases-and-salts-class-10-ppt
- acids-bases-and-salts-pdf
- acids-bases-and-salts-questions-pdf
- acids-bases-and-salts-pyqs
- acids-bases-and-salts-class-10-qna
- class-x-acids-bases-and-salts-pdf
- class-x-acids-bases-and-salts
- acid-bases-and-salts-class-x-pdf
- acids-bases-salts-class-7-worksheet
- science-ch-2-acids-bases-and-salts
- acid-bases-salts-10
- acids-bases-and-salts-chart
- acid-bases-salts-notes
- chapter-1-:-acids-bases-and-salts-summary
- acids-bases-and-salts-1
- chapter-2-acids-bases-and-salts-notes
- chapter-2-acids-bases-and-salts-pdf
- class-10-chapter-2-acids-bases-and-salts
- acids-bases-and-salts-3-marks-questions
- chapter-4-acids-bases-and-salts-class-7
- class-7-science-ch-4-acids-bases-and-salts
- chapter-4-acids-bases-and-salts
- acids-bases-and-salts-form-4
- chapter-5-acids-bases-and-salts
- class-6-acids-bases-and-salts-pdf
- acids-bases-and-salts-class-6
- acids-bases-and-salts-class-7-notes
- acids-bases-and-salts-class-7-pdf
- acids-bases-and-salts-class-7-mcq
- acids-bases-and-salts-class-7-worksheet
- acids-bases-and-salts-class-8
- acids-bases-and-salts-class-9
- acids-bases-salts
- is-salt-an-acid-or-a-base
- what-is-acid-base-and-salt
- what-are-salts-acids-and-bases
- is-salt-acid-or-base
- is-salt-an-acid-or-base
- acids-bases-salts
- important-question-of-acid-bases-and-salt
- difference-between-acids-bases-and-salts
- notes-for-acid-bases-and-salts
- acid-plus-base-equals-salt-plus-water
- important-topics-in-acid-bases-and-salt
- difference-between-acid-base-and-salt
- properties-of-acids-bases-and-salts
- acids-bases-salts
- acids-bases-salts-notes
- notes-on-acids-bases-and-salts
- acids-bases-and-salts-examples
- acids-bases-and-salts-class-10-notes-aesthetic
- chemistry-acids-bases-and-salts-project
- acids-bases-and-salts-aesthetic
- acids-bases-and-salts-notes-class-7
- acids-bases-and-salts-poster
- acids-,-bases-and-salts
- acids-bases-and-salts-tlm
- acids-bases-and-salts-class-10
- acids-bases-and-salts-class-7-mind-map
- acids-bases-and-salts-chemistry
- acids-bases-and-salts-class-10-mindmap
- chemistry-acids-bases-and-salts-notes
- acids-bases-and-salts-class-10-short-notes
- acids-bases-and-salts-class-10-concept-map
- acids-bases-and-salts-diagrams
- acids-bases-and-salts-design
- acids-bases-and-salts-drawings
- acids-bases-and-salts-front-page
- acids-bases-and-salts-heading-ideas
- acids-bases-and-salts-heading
- mind-map-of-acids-bases-and-salts
- acids-bases-and-salts-model
- acids-bases-and-salts-mindmap
- acids-bases-and-salts-mind-map-class-10
- acids-bases-and-salts-class-10-notes-mind-map
- mind-map-of-acids-bases-and-salts-class-10
- acids-bases-and-salts-notes-class-10th
- acids-bases-and-salts-notes
- acids-bases-and-salts-notes-aesthetic
- class-10-acids-bases-and-salts-notes
- poster-on-acids-bases-and-salts
- acids-bases-and-salts-one-page-notes
- chart-on-acids-bases-and-salts
- reactions-of-acids,-salts-and-bases
- acids-bases-and-salts-project
- acids-bases-and-salts-title-page
- acids-bases-and-salts-picture
- acids-bases-and-salts-textbook
- acids-bases-and-salts-worksheet
- acids-bases-and-salts-class-10-chart
- acids-bases-and-salts-class-10-notes-chemistry
- acids-bases-and-salts-class-7
- chapter-2-acids-bases-and-salts
- cbse