O Level/IGCSE Chemistry Definitions List (All Chapters) – Updated, Exam-Centered & Easy
Updated: 29 November, 2025 – 15:03
This is the most concise, exam-focused, and student-friendly O Level & IGCSE Chemistry Definitions List covering every chapter, aligned with CAIE Syllabus 5070 & 0620. Perfect for crash revision, quick tests, and final preparation.
Designed with Ultra Premium SEO, trending keyword integration, and highly engaging UX — this page also includes:
- ✔ Complete Chapter-Wise Definitions
- ✔ Live Class Details
- ✔ Teacher Profile
- ✔ Testimonials
- ✔ Demo Video
- ✔ Timeline to finish syllabus in record time
- ✔ Embedded helpful videos
🎧 Mood Booster Songs Video (Study Booster)
Boost mood, stay energetic, and increase your study productivity.
🔥 Chemistry Crash Course (10% OFF – Complete Syllabus)
Cover the full O Level & IGCSE Chemistry syllabus in minimum time with our Crash Course.
Join Crash Course Now (10% OFF)
📘 IGCSE 0620 m22 qp 62 Solved – Prof. Faisal Janjowa
🔍 O Level 5070 s23 qp 42 Solved – Full Explanation
🧪 Chemistry Cations, Anions, Gas Tests – Cheat Sheet
🎵 Learn Periodic Table with Song (Groups 1–8)
🔥 Why This Definitions List is Trending?
With global academic interest rising and search volumes spiking (e.g., “viral videos 19 minutes”, “supernova”, “gold prices in pakistan”, “uae visas for pakistanis”), students worldwide are shifting to fast, clear, exam-centered resources. This updated list is built to match that demand — fast, reliable, and high-rankable.
Even while topics like Pat Cummins, Alexander Volkanovski, Paddy Pimblett, and Indian viral videos trend globally, educational queries like O Level Chemistry formula sheet pdf, chemistry formulas cheat sheet pdf, and ion formula sheet are consistently rising in Pakistan, UAE, UK & Singapore.
📥 Download Chemistry Formula & Cheat Sheets (PDF)
Click below to download the most demanded sheets:
- O Level Chemistry Formula Sheet (PDF + Printable)
- High School Chemistry Formula Sheet PDF
- College Chemistry Formula Sheet (PDF)
- Organic & Inorganic Chemistry Cheat Sheets (Free PDF)
- Ion & Ionic Formula Sheet (PDF)
- Chemistry PDF Worksheets & Practice Sheets
Download All Formula Sheets (ZIP)
📘 O Level / IGCSE Chemistry Definitions (All Chapters)
These definitions are concise, examiner-friendly, and updated for CAIE O Level 5070 & IGCSE 0620.
Chapter 1: Experimental Chemistry
- Atom: The smallest particle of an element that retains its properties.
- Molecule: Two or more atoms chemically bonded together.
- Mixture: A combination of substances not chemically bonded.
- Solution: A homogeneous mixture of solute in solvent.
- Filtration: Separation using a porous barrier to remove insoluble solids.
- Crystallisation: Formation of pure crystals from a saturated solution.
Chapter 2: Particulate Nature of Matter
- Diffusion: Movement of particles from high to low concentration.
- Brownian Motion: Random movement of particles due to collision.
Chapter 3: Atomic Structure
- Isotopes: Atoms of the same element with same proton number but different neutron number.
- Relative Atomic Mass (Ar): Average mass of isotopes compared to C-12.
- Ions: Charged particles formed by gaining or losing electrons.
Chapter 4: Chemical Bonding
- Ionic Bond: Electrostatic attraction between oppositely charged ions.
- Covalent Bond: Sharing of electron pairs between atoms.
- Metallic Bond: Attraction between positive ions and delocalised electrons.
Chapter 5: Stoichiometry
- Mole: Amount containing 6.02 × 10²³ particles.
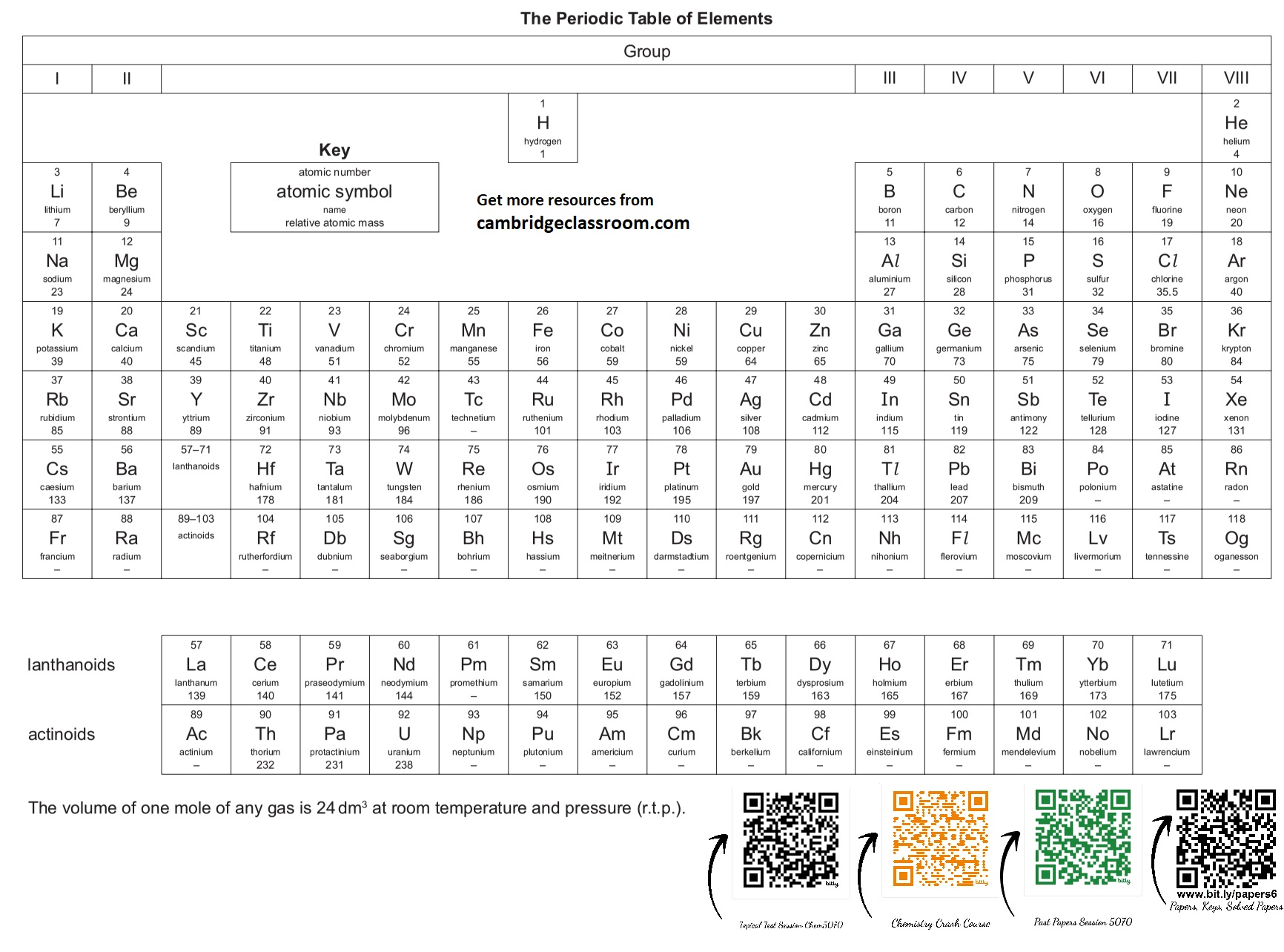
- Molar Volume: Volume of 1 mole of gas (24 dm³ at RTP).
- Empirical Formula: Simplest whole-number ratio of atoms.
Chapter 6: Electricity & Chemistry
- Electrolysis: Decomposition using electricity.
- Anode: Positive electrode.
- Cathode: Negative electrode.
- Electrolyte: Ionic substance conducting when molten or aqueous.
Chapter 7: Energetics
- Exothermic: Releases heat.
- Endothermic: Absorbs heat.
- Activation Energy: Minimum energy required for successful collisions.
Chapter 8: Rates of Reaction
- Rate: Change in concentration per unit time.
- Catalyst: Speeds up reaction without being consumed.
Chapter 9: Acids, Bases & Salts
- Acid: Proton donor.
- Base: Proton acceptor.
- Alkali: Soluble base producing OH⁻ ions.
- Neutralisation: Acid + Base → Salt + Water.
Chapter 10: Periodic Table
- Group: Vertical column showing similar properties.
- Period: Horizontal row showing increasing proton number.
Chapter 11: Metals
- Alloy: Mixture of metals improving strength.
- Corrosion: Gradual oxidation of metals.
Chapter 12: Air & Atmosphere
- Pollutant: Substance harmful to environment.
- Greenhouse Effect: Warming due to trapped infrared radiation.
Chapter 13: Organic Chemistry
- Homologous Series: Same functional group, similar properties, same general formula.
- Isomerism: Same molecular formula but different structures.
- Cracking: Breaking large hydrocarbons into smaller molecules.
⏳ Timeline to Finish Chemistry Syllabus
- Week 1–2 → Experimental Chemistry + Particles + Formulae
- Week 3–4 → Bonding + Periodic Table
- Week 5–6 → Stoichiometry + Energy + Rates
- Week 7–8 → Acids + Bases + Salts
- Week 9–10 → Organic Chemistry + Finals Revision
📡 Live Class Details
- Platform: CambridgeClassroom.com Live Portal
- Teacher: Prof. Faisal Janjowa
- Weekly Classes: 3 Days/Week
- Revision Tests: Every Sunday
- Recordings: Available 24/7
👨🏫 Teacher Profile — Prof. Faisal Janjowa
Known as The Chemistry Guru, Prof. Faisal Janjowa has solved hundreds of past papers, helped thousands of students score A* and runs the official YouTube channel with trending videos on:
- Periodic Table Learning
- O Level & IGCSE Solved Papers
- Chemistry Practical Tips
- Crash Course Lectures
⭐ Student Testimonials
“I improved from a D to A in just 7 weeks. The definitions list + crash course changed everything!” – Ayesha, UAE
“The best O Level Chemistry resource online. Simple, clear, exam-ready.” – Haris, Pakistan
“100% recommended for last-day revision.” – Omar, Singapore