Mole Concept Notes PDF | Acids, Bases & Salts – Complete Exam-Focused Chemistry Guide (IGCSE 0620, O Level 5070, AQA, Edexcel, Cambridge A Level)
Searching endlessly for Mole Concept Notes PDF, Acids, Bases & Salts notes, or IGCSE Chemistry 0620 / O Level Chemistry 5070 explanations on YouTube often leads to confusion, advertisements, incomplete syllabus coverage, and wasted hours.
Just like a live cricket match between sri lanka national cricket team vs pakistan national cricket team match scorecard decides winners by strategy—not randomness—Chemistry success also demands a structured, syllabus-aligned plan.
Why Acids, Bases & Salts + Mole Concept Decide Your Grade
- Over 35–40% exam weightage in IGCSE 0620 & O Level 5070 depends on Mole calculations, acids, bases, salts & reactions
- Foundation for Stoichiometry, Empirical formulas, Redox & Electrolysis
- Repeated MCQs, structured questions & ATP practicals
Random videos—like scrolling PUBG after a 4.2 update pubg mobile—may feel productive but don’t win exams.
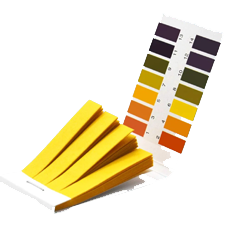
Define Acids, Bases & Salts (Exam-Centered Definitions)
- Acid: A substance that produces H⁺ ions in aqueous solution (e.g. HCl, H₂SO₄)
- Base: A substance that produces OH⁻ ions in aqueous solution (e.g. NaOH, KOH)
- Salt: A compound formed when an acid reacts with a base (e.g. NaCl, CuSO₄)
Aligned with Official Cambridge Definitions.
Why YouTube Fails for Chemistry Exams
| YouTube Videos | CambridgeClassroom Courses |
|---|---|
| Unaligned topics | 100% syllabus-mapped (0620, 5070, AQA, Edexcel) |
| Ads & distractions | Zero ads, pure learning |
| No exam strategy | Past paper + examiner logic |
| Random pacing | Time-optimized lessons |
🚀 Two Smart Ways to Master Acids, Bases & Salts
⚡ Option 1: Master in Just 30 Minutes
Short Course:
Acids, Bases & Salts – Properties & Preparations (30 Minutes)
Perfect for: IGCSE 0620 | O Level 5070 | Class 7–10 | Rapid Revision
🔥 Option 2: Complete Chemistry in ~8 Hours
Full Syllabus Chemistry Crash Course (10% OFF)
Covers Mole Concept, Acids Bases Salts, Electrolysis, Organic Chemistry, Redox & ATP Practicals.
📥 Download Free Notes PDFs (Exam-Focused)
- Moles – PDF
- Acids, Bases & Salts – PDF
- Formula Sheet – PDF
- Electrolysis – PDF
- Organic Chemistry – PDF
More notes: Complete O Level Notes | Save My Exams – IGCSE
🎥 Must-Watch Exam Videos
Mood Booster Study Music
Chemistry Crash Course Ad
IGCSE 0620 Solved Paper
O Level 5070 Solved Paper
Periodic Table Song
👨🏫 Teacher Profile
Professor Faisal Janjowa – “The Chemistry Guru” Cambridge Examiner-style teaching, trusted by thousands of students across Pakistan, UAE, Sri Lanka & UK.
⭐ Student Reviews & Testimonials
“Like watching pak vs sri lanka with expert commentary – suddenly Chemistry made sense.” — IGCSE Student
“I stopped wasting time like scrolling PUBG or Netflix Stranger Things. This course saved my grade.” — O Level 5070 Student
❓ FAQs – Mole Concept & Acids Bases Salts
Q: Are these notes enough for exams?
A: Yes. Fully aligned with CAIE, Edexcel & AQA syllabi.
Q: Can I study without YouTube?
A: Absolutely. Structured content beats random videos.
Q: Suitable for Class 7–10?
A: Yes – concepts scale from basics to A Level.
🚀 Final Call-To-Action
Stop learning Chemistry like random highlights of perth scorchers vs renegades. Start learning like a champion.
👉 Master Acids, Bases & Salts in 30 Minutes
👉 Complete Chemistry Crash Course (10% OFF)
- sri-lanka-national-cricket-team-vs-pakistan-national-cricket-team-match-scorecard
- perth-scorchers-vs-renegades
- moeen-ali
- shadab-khan
- fbise-hssc-2nd-annual-result
- 4.2-update-pubg-mobile
- sassuolo-vs-juventus
- west-ham-vs-nottm-forest
- pak-vs-srilanka
- nasir-hossain
- pubg
- realme-16-pro-5g
- wanindu-hasaranga
- live-cricket-match
- chattogram
- emily-in-paris
- salman-ali-agha
- chattogram-royals-vs-sylhet-titans
- pak-vs-aus
- sri-lanka-vs-pakistan
- shadab-khan
- moeen-ali
- كلباء-ضد-الوصل
- jana-nayagan
- عيدروس-الزبيدي
- uae-citizenship-companies
- sri-lanka-vs-pakistan
- نور
- هيئة-الغذاء-والدواء
- ساحل-العاج-ضد-بوركينا-فاسو
- عيدروس-الزبيدي
- shadab-khan
- perth-scorchers-vs-renegades
- moeen-ali
- noor
- حليب-نان
- نظام
- نتائج
- برشلونة-ضد-أتلتيك-بيلباو
- كلباء-ضد-الوصل
- saudi-arabia
- قسد
- عبير-شمس-الدين
- الهلال-والحزم
- باتريس-لومومبا
- lakers-vs-pelicans
- harbaugh
- michael-reagan
- jordan-shipley
- matt-kalil
- aldrich-ames
- demond-williams
- cavaliers-vs-pacers
- spurs-vs-grizzlies
- netflix-stranger-things-conformity-gate
- heat-vs-timberwolves
- ringer
- kliff-kingsbury
- christopher-judge
- sri-lanka-vs-pakistan
- school-closings-massachusetts
- duke-vs-louisville
- andrea-yates
- philip-yancey
- todd-monken
- paul-feig
- russian-oil-tanker
- hulu
- broadway-week-2026
- tcu-vs-kansas
- checker-tobi-todesfall
- unwetterwarnung-schnee
- wetter-schneefall-nrw
- caro-daur
- loredana-wollnys
- stranger-things-countdown
- schafe-im-supermarkt
- ukraine-trump
- periotrap-zahnpasta
- rentenanpassung-2026
- conformity-gate
- siemens-aktie
- regenradar
- upamecano
- biathlon-oberhof
- verkehr-nrw
- marinera
- bayern-millionen
- sri-lanka-vs-pakistan
- windrad-a44
- nordatlantik
- schneesturm-hamburg
- freudenberg
- alisha-lehmann
- acids,-bases-&-salts
- acids-bases-and-salts-worksheet-with-answers-pdf
- acids-bases-and-salts-worksheet-with-answers-pdf-class-7
- acids-bases-and-salts-worksheet-with-answers-pdf-class-10
- define-acids-bases-and-salts-with-example
- acids-bases-and-salts-examples-with-answers
- acids-bases-and-salts-worksheet-with-answers-pdf-grade
- acids-bases-and-salts-worksheet-with-answers
- acids-bases-and-salts-worksheet-with-answers-class-7
- define-acids-bases-and-salts-with-two-examples-each
- acid-bases-salts-in-hindi
- acid-base-salt-in-hindi-meaning
- acid-base-salt-in-chemistry
- acid-base-salt-for-class-7
- acids-bases-in-salts
- acids-bases-and-salts-for-class-10
- acids-bases-and-salts-in-telugu
- acids-bases-and-salts-for-class-10-notes
- acids-bases-and-salts-for-class-10-pdf
- acids-bases-and-salts-for-class-10-mcq
- acids-bases-and-salts-by-prashant-kirad
- acids-bases-and-salts-in-hindi
- acids-bases-and-salts-in-hindi-meaning
- acids-bases-and-salts-in-chemistry
- acids-bases-and-salts-in-class-7
- acids-bases-and-salts-in-science
- acid-bases-and-salt-of-class-10th
- acid-base-and-salt-in-hindi-pdf
- acid-base-and-salt-in-hindi-class-7
- acids-bases-and-salts-in-kannada
- acids-bases-and-salts-in-kannada-meaning
- uses-of-acids-bases-and-salts-in-our-daily-life
- acids-bases-and-salts-for-class-7
- acids-bases-and-salts-for-jss3
- acids-bases-and-salts-for-grade-7
- acids-bases-and-salts-for-class-7-pdf
- acids-bases-and-salts-for-class-7th
- acids-bases-and-salts-for-class-10-icse
- acid-base-salt-with-example
- acids-bases-and-salts-in-daily-life
- acids-bases-and-salts-in-tamil
- acids-bases-and-salts-in-calligraphy
- acids-bases-and-salts-in-text-questions
- acids-bases-and-salts-in-marathi
- acid-base-salt-in-tamil
- acid-base-salt-in-bengali
- acid-base-salt-in-our-daily-life
- acid-base-to-salt-water
- acids-bases-and-salts-past-papers
- acid-base-salt-plus-water
- acids-plus-bases
- acids-bases-and-salts-pre-lab
- acid-base-and-salt-with-example
- unlike-bases-acids-will-cause-what-to-dissolved
- acids-and-bases-have-many-uses-around-the
- acid-bases-and-salts-class-10-up-board
- acid-bases-and-salts-class-10-notes-up-board
- acids-and-bases-vs-salts
- acids-versus-bases
- acids,-bases-&-salts
- are-acids-bases-and-salts-ionic-compounds
- what-are-acids-bases-salts
- what-are-acids-bases-and-salts-worksheet
- what-are-the-uses-of-acids-bases-and-salts
- how-are-salts-related-to-acids-and-bases
- what-are-acids-bases-and-salts-with-examples
- what-are-the-characteristics-of-acids-bases-and-salts
- what-are-the-properties-of-acids-bases-and-salts
- what-are-acids-bases-and-salts-in-chemistry
- what-are-the-acids-bases-and-salts
- can-acids-and-bases-mix
- can-acids-and-bases-mix-together
- can-bases-be-salts
- can-acids-and-bases-be-stored-together
- can-acids-be-bases
- do-acids-bases-and-salts-conduct-electricity
- how-do-acids-bases-and-salts-differ
- do-all-bases-form-salts-with-acids
- do-acids-and-bases-form-salts
- how-do-the-properties-of-acids-bases-and-salts-compare
- what-do-acids-bases-and-salts-have-in-common
- do-acids-react-with-bases-to-form-salts
- what-do-you-mean-by-acids-bases-and-salts
- how-do-acids-bases-and-salts-compare
- does-acids-or-bases-conduct-electricity
- do-acids-and-bases-react-with-each-other
- what-property-do-acids-bases-and-salts-have-in-common
- what-acids-and-bases-have-in-common
- how-acids-bases-and-salts-behave-in-water
- how-to-identify-acids-bases-and-salts
- how-to-differentiate-between-acids-bases-and-salts
- how-to-classify-compounds-as-acids-bases-or-salts
- how-acids-and-bases-work
- how-are-acids-and-bases-important
- how-are-acids-and-bases-used
- is-acids-bases-and-salts
- what-is-the-difference-between-acids-bases-and-salts
- what-is-the-meaning-of-acids-bases-and-salts
- what-is-acids-bases-and-salts-class-10
- what-is-acids-bases-and-salts-in-chemistry
- what-is-acids-bases-and-salts-class-7
- what-is-the-hindi-meaning-of-acids-bases-and-salts
- how-should-acids-and-bases-be-stored
- why-should-acids-and-bases-not-be-stored-together
- how-do-acids-bases-and-salts-affect-your-daily-life
- what-are-acids-salts-and-bases
- what-are-acids-bases-and-salts
- when-acids-and-bases-are-mixed
- when-acids-and-bases-react-what-are-the-products
- where-are-acids-and-bases-commonly-used
- where-are-acids-and-bases-found
- where-are-acids-and-bases-located
- where-are-acids-and-bases-found-in-the-body
- where-can-you-find-acids-and-bases-in-everyday-life
- which-acids-and-bases-are-strong
- which-salts-are-acidic
- which-salts-are-basic
- what-are-acids-and-bases-used-for
- what-acids-and-bases-are
- why-do-acids-bases-and-salts-conduct-electricity
- why-are-acids-bases-and-salts-electrolytes
- why-are-acids-and-bases-important-in-chemistry
- why-are-acids-and-bases-important-in-biology
- why-are-acids-and-bases-important
- why-are-acids-and-bases-important-in-our-lives
- will-acids-and-bases-neutralize-each-other
- what-can-acids-and-bases-do
- what-are-acids-bases-and-salts-worksheet-answers
- how-can-acids-and-bases-be-dangerous
- acids,-bases-&-salts
- class-10-science-acids-bases-salts
- acids-bases-and-salts
- acids-bases-and-salts-worksheet
- acids-bases-and-salts-worksheet-with-answers-pdf
- acids-bases-and-salts-word-search-answers
- acids-bases-and-salts-class-10
- acids-bases-and-salts-class-10-notes
- acids-bases-and-salts-class-7
- acids-bases-and-salts-class-10-pdf
- acids-bases-and-salts-notes-pdf
- acids-bases-and-salts-class-7-pdf
- acids-bases-salts-activities
- acid-base-salt-and-water
- acid-base-salt-and
- acid-base-salt-all-formula
- acid-base-salt-and-indicator
- acid-base-salt-assignment
- acids-bases-and-salts-class-7-questions-and-answers-pdf
- acids-bases-and-salts-class-10-questions-and-answers-pdf
- acids-bases-and-salts-class-10-notes-pdf-download
- acids-bases-and-salts-notes-pdf-download
- acids-bases-and-salts-bank-of-biology
- acids-bases-and-salts-board-questions
- acids-bases-and-salts-bbc-bitesize
- acids-bases-and-salts-book
- acids-bases-and-salts-book-pdf
- acids-bases-and-salts-bits
- acids-bases-and-salts-byjus
- acids-bases-and-salts-byju’s-class-7
- acids-bases-and-salts-byju’s-class-10
- competency-based-questions-on-acids-bases-and-salts-class-10
- bank-of-biology-class-10-acids-bases-and-salts
- case-based-questions-on-acids-bases-and-salts-class-10
- case-based-questions-on-acids-bases-and-salts-class-7
- ncert-science-book-class-10-acids-bases-and-salts
- ncert-science-book-class-7-acids-bases-and-salts
- differentiate-between-acids-bases-and-salts
- acids-bases-and-salts-class-10-ncert-pdf
- acids-bases-and-salts-class-10-pyq
- class-10-acids-bases-salts-notes
- class-10-acids-bases-salts
- class-10-acids-bases-salts-pdf
- class-10-acids-bases-salts-mcq
- class-10-acids-bases-salts-solutions
- class-7-acids-bases-salts
- chemistry-acids-bases-salts
- class-10-acids-bases-salts-ncert-solutions
- acids-bases-salts-definition
- acids-bases-salts-diagram
- acid-base-salt-difference
- acid-base-salt-drawing
- acid-base-salt-dash
- acid-base-salt-define
- acid-base-salt-definition-class-10
- acid-base-salt-definition-with-example
- acids-bases-and-salts-diagram-class-7
- acids-bases-and-salts-drawing-easy
- difference-between-acids-bases-salts
- define-acids-bases-salts
- class-7-acids-bases-and-salts-notes-pdf-download
- acids-bases-and-salts-class-10-pdf-download
- acids-bases-and-salts-class-10-ppt-free-download
- acids-bases-and-salts-definition
- acids-bases-and-salts-class-7-ppt-free-download
- acids-bases-and-salts-notes-pdf-free-download
- acids-bases-salts-exemplar
- acids-bases-salts-extra-questions
- acids-bases-salts-examples
- acid-bases-salts-exercise
- acid-base-salt-extra-questions-class-10
- acid-base-salt-extra-questions-class-7
- acid-base-salt-experiment
- acid-base-salt-explanation
- acid-base-salt-equation
- acid-base-salt-extra-question-answer
- examples-of-acids-bases-salts
- acids-bases-and-salts-class-7-extra-questions-and-answers
- class-7-acids-bases-and-salts-extra-questions
- acids-bases-and-salts-class-10-extra-questions
- acids-bases-and-salts-extra-questions
- class-7-science-acids-bases-and-salts-extra-questions
- ncert-exemplar-class-10-acids-bases-and-salts-solutions
- ncert-exemplar-class-7-science-acids-bases-and-salts
- acids-bases-and-salts-class-10-exercise
- ncert-exemplar-class-10-acids-bases-and-salts
- acids-bases-and-salts-formulas
- acids-bases-and-salts-form-4-notes
- acids-bases-and-salts-file-type-ppt
- acids-bases-and-salts-for-class-7
- acids-bases-and-salts-for-jss3
- acids-bases-and-salts-flow-chart
- acids-bases-and-salts-for-class-10
- acids-bases-and-salts-formula-sheet
- acids-bases-and-salts-front-page
- acids-bases-and-salts-for-class-10-notes
- questions-on-acids-bases-and-salts-for-class-7
- important-questions-from-acids-bases-and-salts
- notes-for-acids-bases-and-salts
- important-questions-from-acids-bases-and-salts-class-10
- ncert-solutions-for-class-10-acids-bases-and-salts
- mcq-from-acids-bases-and-salts-class-10
- notes-for-acids-bases-and-salts-class-10
- notes-for-class-10-science-acids-bases-and-salts
- acids-bases-and-salts-grade-7-questions
- acids-bases-and-salts-grade-10
- acids-bases-and-salts-grade-7
- acids-bases-and-salts-gcse-chemistry
- acids-bases-and-salts-gcse-questions
- acids-bases-and-salts-gcse
- acids-bases-and-salts-grade-10-pdf
- acids-bases-and-salts-grade-7-extra-questions
- acids-bases-and-salts-grade-7-ppt
- acids-bases-and-salts-grade-7-notes
- acids-bases-and-salts-questions-and-answers-pdf-gcse
- grade-7-acids-bases-and-salts
- give-a-few-uses-of-acids-bases-and-salts-respectively
- acids-bases-and-salts-class-10-ppt-google-docs
- grade-10-acids-bases-and-salts-notes
- grade-7-acids-bases-and-salts-assertion-reason-questions
- grade-7-acids-bases-and-salts-extra-questions
- lesson-plan-on-acids-bases-and-salts-for-grade-7
- grade-10-acids-bases-and-salts-pdf
- acid-base-salt-hindi
- acid-base-salt-hindi-meaning
- acid-base-salt-hydrogen
- acid-base-salt-h2o
- acid-base-and-salts-handwritten-notes
- acid-base-salt-hydrogen-gas
- acid-base-salt-hindi-notes
- acid-base-salt-hard-questions
- acid-base-salt-h2
- acid+base=-salt-h20
- acids-bases-and-salts-meaning-in-hindi
- acids-bases-and-salts-in-hindi
- class-10-acids-bases-and-salts-handwritten-notes
- hots-questions-on-acids-bases-and-salts-class-7
- hots-questions-on-acids-bases-and-salts-class-10
- acids-bases-and-salts-handwritten-notes
- acids-bases-and-salts-class-10-pdf-notes-handwritten
- class-10-acids-bases-and-salts-notes-in-hindi
- hots-questions-on-acids-bases-and-salts
- acids-bases-and-salts-important-questions
- acids-bases-and-salts-igcse
- acids-bases-and-salts-important-questions-class-10
- acids-bases-and-salts-igcse-questions
- acids-bases-and-salts-igcse-notes
- acids-bases-and-salts-is-chemistry-or-physics
- acids-bases-and-salts-images
- acids-bases-and-salts-in-telugu
- acids-bases-and-salts-intext-questions
- igcse-chemistry-acids-bases-salts
- acids-bases-and-salts-class-10-icse
- acids-bases-and-salts-class-10-intext-questions
- acids-bases-and-salts-class-10-icse-notes
- acids-bases-and-salts-jss3
- acids-bases-and-salts-jss3-basic-science
- acids-bases-and-salts-jss3-notes
- acids-bases-and-salts-jee
- acid-base-and-salt-jss3-lesson-note
- acid-base-and-salt-jss3-basic-science-notes
- acid-base-and-salt-jss3-basic-science-pdf
- acid-base-and-salt-jamb
- acid-base-and-salt-jss3-lesson-note-pdf
- acid-base-and-salt-js3
- acids-bases-and-salts-class-7-jkbose
- acids-bases-and-salts-class-7-questions-and-answers-jkbose
- acids-bases-and-salts-class-7-jkbose-solutions
- acids-bases-and-salts-iit-jee-pdf
- jamb-questions-on-acids-bases-and-salts
- acids-bases-and-salts-iit-jee-questions
- acids-bases-and-salts-class-10-notes-jagran-josh
- acids-bases-and-salts-class-10-jkbose
- acid-base-salt-ka-hindi
- acid-base-salt-kya-hai
- acid-base-salt-ki-definition
- acid-base-salt-ka-diagram
- acid-base-and-salts-ka-notes
- acid-base-salt-ka-example
- acid-base-salt-ka-formula
- acid-base-salt-ka-hindi-meaning
- acid-base-salt-ka
- acids-bases-and-salts-key-points
- acids-bases-and-salts-class-10-notes-prashant-kirad
- kseeb-solutions-for-class-7-science-acids-bases-and-salts
- prashant-kirad-acids-bases-and-salts-notes
- kseeb-solutions-for-class-10-science-acids-bases-and-salts
- acids-bases-and-salts-class-10-prashant-kirad
- prashant-kirad-acids-bases-and-salts
- kseeb-solutions-for-class-10-acids-bases-and-salts
- acids-bases-and-salts-class-10-key-points
- acids-bases-and-salts-class-9-samacheer-kalvi
- acids-bases-and-salts-lesson
- acids-bases-and-salts-lab-report
- acids-bases-and-salts-list
- acids-bases-and-salts-lesson-plan-class-10
- acids-bases-and-salts-lesson-plan
- acids-bases-and-salts-lesson-pdf
- acids-bases-and-salts-lesson-explanation
- acids-bases-and-salts-learn-cbse
- acids-bases-and-salts-long-questions
- acids-bases-and-salts-lacking-carbon-are
- lab-14-acids-bases-salts-and-buffers
- 10th-class-acids-bases-and-salts-lesson
- lesson-plan-for-acids-bases-and-salts-class-10
- learn-cbse-class-7-science-acids-bases-and-salts
- learn-cbse-class-10-acids-bases-and-salts
- acids-bases-and-salts-class-7-mcq-learn-insta
- learning-outcomes-of-acids-bases-and-salts-class-7
- uses-of-acids-bases-and-salts-in-our-daily-life
- acids-bases-and-salts-mcq
- acids-bases-and-salts-mcq-class-10
- acids-bases-and-salts-mind-map
- acids-bases-and-salts-mcq-class-7
- acids-bases-and-salts-mind-map-class-10
- acids-bases-and-salts-meaning-in-urdu
- acids-bases-and-salts-meaning
- acids-bases-and-salts-mcq-class-10-pdf
- acids-bases-and-salts-mcq-test
- mcq-acids-bases-salts-class-10
- mcq-on-acids-bases-salts-class-7
- mcq-on-acids-bases-and-salts-class-10-pdf
- multiple-choice-questions-on-acids-bases-and-salts-pdf
- mcq-on-acids-bases-and-salts-class-7-pdf
- acids-bases-and-salts-class-7-mcq-online-test
- acids-bases-and-salts-class-10-mcq-online-test
- mcq-questions-on-acids-bases-and-salts-class-10
- acids-bases-salts-notes
- acids-bases-salts-ncert
- acids-bases-salts-ncert-solutions
- acids-bases-salts-notes-class-10
- acids-bases-salts-ncert-pdf
- acids-bases-salts-notes-pdf
- acids-bases-salts-ncert-exemplar
- acid-bases-salts-ncert-class-10
- acid-base-salts-ncert-question-answer
- acid-base-salt-notes-class-10-pdf
- notes-of-chemistry-class-10-acids-bases-salts
- ncert-acids-bases-salts
- ncert-solutions-class-10-acids-bases-salts
- ncert-class-10-acids-bases-salts-pdf
- ncert-class-10-acids-bases-salts
- acids-bases-and-salts-notes
- acids-bases-and-salts-class-7-notes
- acids-bases-and-salts-one-shot
- acids-bases-and-salts-o-level-notes
- acids-bases-and-salts-organic-chemistry-tutor
- acids-bases-and-salts-o-level-questions
- acids-bases-and-salts-o-level-past-papers
- acids-bases-and-salts-o-level
- acids-bases-and-salts-objective-questions
- acids-bases-and-salts-o-level-chemistry
- acids-bases-and-salts-o-level-notes-pdf
- acids-bases-and-salts-objectives
- are-salts-acids-or-bases
- notes-of-acids-bases-and-salts
- notes-on-acids-bases-and-salts-class-7
- ncert-solutions-of-acids-bases-and-salts-class-10
- questions-on-acids-bases-and-salts-class-10-with-answers
- questions-and-answers-on-acids-bases-and-salts-class-7
- notes-on-acids-bases-and-salts-pdf
- acids-bases-and-salts-pdf
- acids-bases-and-salts-pyqs
- acids-bases-and-salts-ppt
- acids-bases-and-salts-pyqs-class-10
- acids-bases-and-salts-previous-year-questions
- acids-bases-and-salts-pdf-class-10
- acids-bases-and-salts-project
- acids-bases-and-salts-pmt
- acids-bases-and-salts-past-papers
- acids-bases-and-salts-ppt-class-10
- properties-of-acids-bases-salts
- acids-bases-salts-questions
- acids-bases-salts-quiz
- acids-bases-salts-quiz-questions
- acids-bases-salts-question-answers
- acid-bases-salts-questions-class-10
- acid-base-salt-question-answer-class-10
- acid-base-salt-question-answer-class-7
- acid-base-salt-question-answer-class-10th
- acid-base-and-salts-question-paper
- acid-base-salt-question-pdf
- acids-bases-and-salts-class-10-questions-and-answers
- acids-bases-and-salts-questions-and-answers-pdf
- acids-bases-and-salts-class-7-questions-and-answers
- acids-bases-and-salts-reactions
- acids-bases-and-salts-rapid-revision
- acids-bases-and-salts-revision-questions
- acids-bases-and-salts-rationalised-pdf
- acids-bases-and-salts-revision-notes
- acids-bases-and-salts-revision
- acids-bases-and-salts-running-notes
- acids-bases-and-salts-report-sheet
- acids-bases-and-salts-regents-questions
- acids-bases-and-salts-review
- assertion-reason-questions-on-acids-bases-and-salts-class-7
- all-reactions-of-acids-bases-and-salts-class-10
- acids-bases-and-salts-class-10-notes-study-rankers
- acids-bases-and-salts-class-7-assertion-and-reason
- acids-bases-and-salts-class-10-assertion-and-reason
- assertion-reason-questions-on-acids-bases-and-salts-class-10
- acids-bases-and-salts-class-10-study-rankers
- acids-bases-and-salts-solutions
- acids-bases-and-salts-short-notes
- acids-bases-and-salts-summary
- acids-bases-and-salts-sample-paper
- acids-bases-and-salts-solutions-class-10
- acids-bases-and-salts-std-9
- acids-bases-and-salts-short-notes-class-10
- acids-bases-and-salts-short-notes-pdf
- acids-bases-and-salts-slideshare
- acids-bases-and-salts-save-my-exams
- class-7-science-chapter-4-acids-bases-and-salts
- acids-bases-and-salts-class-10-solutions
- class-7-science-acids-bases-and-salts
- ncert-solutions-acids-bases-and-salts-class-7
- class-7-science-chapter-5-acids-bases-and-salts
- class-7-science-chapter-acids-bases-and-salts
- case-study-questions-on-acids-bases-and-salts-class-7
- acids-bases-and-salts-ncert-solutions
- class-10-science-ch-acids-bases-and-salts
- class-10-science-chapter-acids-bases-and-salts-notes
- acids-bases-and-salts-title-page
- acids-bases-and-salts-test
- acids-bases-and-salts-test-class-10
- acids-bases-and-salts-table
- acids-bases-and-salts-textbook-pdf
- acids-bases-and-salts-their-uses-in-daily-life
- acids-bases-and-salts-test-pdf
- acids-bases-and-salts-teachoo
- acids-bases-and-salts-topical-questions
- acids-bases-and-salts-textbook-questions-and-answers
- the-four-classifications-of-compounds-are-acids-bases-salts-and
- acids-bases-and-salts-class-10-test-paper-pdf
- test-on-acids-bases-and-salts-class-7
- important-topics-in-acids-bases-and-salts-class-10
- acids-bases-and-salts-class-10-textbook-pdf
- acid-base-salt-upsc
- acid-base-salt-uses
- acids-bases-and-salts-used-in-our-daily-life
- acids-bases-and-salts-unacademy
- acids-bases-and-salts-used-in-therapeutic-processes
- acid-base-salt-water-uses
- acid-base-and-salt-upsc-notes
- acids-bases-and-salts-in-urdu
- uses-of-acids-bases-salts
- write-any-three-uses-each-of-acids-bases-and-salts
- uses-of-acids-bases-and-salts-class-7
- acids-bases-and-salts-upsc
- acid-base-salt-video
- acids-bases-and-salts-videos-for-class-7
- acids-bases-and-salts-vedantu
- acid-bases-and-salts-vaga-study
- acid-base-and-salts-very-short-questions
- acid-base-and-salts-very-short-question-answer
- acid-base-salt-ph-value
- acid-base-or-salt-vinegar
- acids-and-bases-vs-salts
- acids-bases-and-salts-class-10-videos-in-english
- vedantu-acids-bases-and-salts
- acids-bases-and-salts-class-10-animated-videos
- video-on-acids-bases-and-salts
- acids-bases-and-salts-class-10-notes-vedantu
- vedantu-class-10-chemistry-acids-bases-and-salts
- ph-value-of-acids-bases-and-salts
- acids-bases-and-salts-class-10-video
- acids-bases-and-salts-worksheet-with-answers-pdf-class-7
- acids-bases-and-salts-worksheet-pdf
- acids-bases-and-salts-worksheet-class-7
- acids-bases-and-salts-worksheet-class-10
- acids-bases-and-salts-worksheet-with-answers-pdf-class-10
- acids-bases-and-salts-wikipedia
- acids-bases-and-salts-wjec
- what’s-in-the-beaker-acids-bases-salts-and-buffers
- what-are-acids-bases-salts
- what’s-in-the-beaker-acids-bases-salts-and-buffers-answers
- write-uses-of-acids-bases-salts
- worksheet-on-acids-bases-and-salts-for-class-7
- igcse-acids-bases-and-salts-worksheet-with-answers-pdf
- acids-bases-and-salts-class-7-worksheet-with-answers-pdf
- questions-on-acids-bases-and-salts-for-class-7-worksheet
- acids-bases-and-salts-xylem
- acids-bases-and-salts-class-x
- acids-bases-and-salts-class-10-xylem
- what-are-the-acids-bases-and-salts
- acids-bases-and-salts-examples
- acids-bases-and-salts-explained
- acids-bases-and-salts-cxc
- acids-bases-and-salts-chemistry
- class-x-acids-bases-and-salts-notes
- class-x-acids-bases-and-salts
- class-x-acids-bases-and-salts-mcq
- class-x-science-acids-bases-and-salts
- class-x-acids-bases-and-salts-pdf
- class-x-acids-bases-and-salts-worksheet
- class-x-science-acids-bases-and-salts-notes
- class-x-acids-bases-and-salts-pyq
- acids-bases-and-salts-youtube
- acid-bases-salts-previous-year-question
- acid-base-salt-previous-year-question-class-10
- acids-bases-and-salts-previous-year-board-questions
- what-are-acids-bases-and-salts-worksheet
- acids-bases-and-salts-class-10-previous-year-questions
- grade-10-acids-bases-and-salts-previous-year-questions
- what-do-you-mean-by-acids-bases-and-salts
- salts-acids-and-bases-examples
- salts-acids-and-bases-chemistry
- znotes-chemistry-acids-bases-and-salts
- acid-bases-and-salts/0620
- acids-bases-and-salts-#1
- 0620-acids-bases-and-salts
- acids-bases-and-salts-organic-chemistry
- acids-bases-and-salts-10th-class
- acids-bases-and-salts-1-mark-questions
- acids-bases-and-salts-1-chemsheets-answers
- acids-bases-and-salts-10-notes
- acids-bases-and-salts-10-pdf
- acids-bases-and-salts-1
- acids-bases-and-salts-1-chemsheets
- acids-bases-and-salts-1-answers
- acids-bases-and-salts-10th-pdf
- acids-bases-and-salts-10
- acids-bases-and-salts-class-10-important-questions
- acids-bases-and-salts-class-10-ppt
- acids-bases-and-salts-class-10-notes-pdf
- ch-acids-bases-and-salts-class-10
- acids-bases-and-salts-2-marks-questions
- acids-bases-and-salts-2-chemsheets-answers
- acids-bases-and-salts-2
- acids-bases-and-salts-activity-2.1
- acids-bases-and-salts-activity-2.2
- acids-bases-and-salts-chapter-2-class-10
- acids-bases-and-salts-activity-2.3
- acids-bases-and-salts-activity-2.8
- acids-bases-and-salts-activity-2.9
- acids-bases-and-salts-table-2.2
- class-10-acids-bases-and-salts-activity-2.3
- 2-acids-bases-and-salts
- ch-2-acids-bases-and-salts-ncert-solutions
- ch-2-acids-bases-and-salts-class-10
- chapter-2-acids-bases-and-salts-important-questions
- chapter-2-acids-bases-and-salts-activity
- chapter-2-acids-bases-and-salts-extra-questions
- chapter-2-acids-bases-and-salts-mcq
- chapter-2-acids-bases-and-salts-notes-pdf
- science-chapter-2-acids-bases-and-salts
- acids-bases-and-salts-3-marks-questions
- acid-base-and-salt-3d-model
- acid-base-and-salt-jss-3
- acid-base-and-salt-form-3
- acids-bases-and-salts-class-10-3-mark-questions
- 3-acids-bases-and-salts
- chapter-3-acids-bases-and-salts
- chapter-3-acids-bases-and-salts-notes
- chapter-3-acids-bases-and-salts-class-10-icse
- chapter-3-acids-bases-and-salts-class-7
- 10th-class-physics-3rd-lesson-acids-bases-and-salts
- unit-test-paper-3a-acids-bases-and-salts
- 3-mark-questions-on-acids-bases-and-salts-class-10
- class-7-science-chapter-3-acids-bases-and-salts
- chemistry-class-10-chapter-3-acids-bases-and-salts
- acids-bases-and-salts-chapter-4-class-7
- acids-bases-and-salts-form-4
- acids-bases-and-salts-table-4.1
- acids-bases-and-salts-chapter-4
- acids-bases-and-salts-table-4.2
- acids-bases-and-salts-table-4.3
- acids-bases-and-salts-table-4.4
- acids-bases-and-salts-table-4.5
- 4-acids-bases-and-salts
- 7th-class-science-4th-lesson-acids-bases-and-salts
- chapter-4-acids-bases-and-salts-class-7
- science-ch-4-acids-bases-and-salts
- chapter-4-acids-bases-and-salts-ncert-solutions
- chapter-4-acids-bases-and-salts-extra-questions
- ch-4-acids-bases-and-salts-notes
- science-chapter-4-acids-bases-and-salts-notes
- chapter-4-acids-bases-and-salts-mcq
- chapter-4-acids-bases-and-salts-exercise
- acids-bases-and-salts-5-mark-questions
- acids-bases-and-salts-5070
- acids-bases-and-salts-chapter-5-class-7
- acids-bases-and-salts-chapter-5
- acids-bases-and-salts-class-10-5-mark-questions
- 5-acids-bases-and-salts
- 5-acids-bases-and-salts-exercise
- 5-acids-bases-and-salts-class-9
- 5-acids-bases-and-salts-exercise-class-9
- 5-acids-bases-and-salts-notes
- 5-acids-bases-and-salts-9th
- 5-acids-bases-and-salts-project
- chapter-5-acids-bases-and-salts-notes
- lesson-5-acids-bases-and-salts
- science-chapter-5-acids-bases-and-salts-mcq
- acids-bases-and-salts-class-6
- acids-bases-and-salts-class-6-notes
- acids-bases-and-salts-grade-6
- experiment-6-acids-bases-and-salts
- experiment-6-acids-bases-and-salts-prelab
- experiment-6-acids-bases-and-salts-report-sheet
- experiment-6-acids-bases-and-salts-lab-report
- experiment-6-prelaboratory-assignment-acids-bases-and-salts
- class-7-science-chapter-6-acids-bases-and-salts
- experiment-6-report-sheet-acids-bases-and-salts
- acids-bases-and-salts-7th-class
- acids-bases-and-salts-7th-class-notes
- acids-bases-and-salts-7th-class-pdf
- acids-bases-and-salts-7th
- acids-bases-and-salts-7
- acids-bases-and-salts-7th-ncert-solutions
- acids-bases-and-salts-7th-science
- acid-base-and-salt-7th-class-question-answer
- acid-base-and-salt-7th-standard
- class-7-acids-bases-and-salts-mcq
- class-7-acids-bases-and-salts-worksheet-pdf
- ncert-class-7-acids-bases-and-salts
- cbse-class-7-acids-bases-and-salts-extra-questions
- class-7-acids-bases-and-salts-solutions
- cbse-class-7-acids-bases-and-salts
- class-7-acids-bases-and-salts-important-questions
- science-class-7-acids-bases-and-salts-notes
- class-7-acids-bases-and-salts-notes-pdf
- acids-bases-and-salts-class-8
- acids-bases-and-salts-class-8-pdf
- acids-bases-and-salts-class-8-notes
- acids-bases-and-salts-class-8-mcq
- acids-bases-and-salts-grade-8
- acids-bases-and-salts-class-8-pdf-notes
- acids-bases-and-salts-class-8-worksheet
- acids-bases-and-salts-class-8-pdf-solutions
- acid-bases-and-salts-class-8-ncert
- acid-base-and-salts-class-8-question-answer
- class-8-chemistry-acids-bases-and-salts
- questions-on-acids-bases-and-salts-for-class-8
- acids-bases-and-salts-9th-class
- acids-bases-and-salts-9th
- acids-bases-and-salts-9
- acid-base-and-salts-class-9th-exercise
- acid-base-and-salt-9th-standard
- acids-bases-and-salts-class-9-notes-pdf
- acids-bases-and-salts-class-9-pdf
- acids-bases-and-salts-class-9-notes
- acids-bases-and-salts-class-9-ssc
- 9th-class-science-acids-bases-and-salts-question-answer
- 9th-standard-science-acids-bases-and-salts
- 5-acids-bases-and-salts-exercise-9th
- 9th-science-acids-bases-and-salts-book-back-answers
- 9th-science-guide-acids-bases-and-salts
- class-9th-science-chapter-5-acids-bases-and-salts
- acids-bases-and-salts-class-9-questions-and-answers-pdf
- acids,-bases-&-salts
- acids-bases-&-salts
- acids-bases-salts
- acids-bases-and-salts-class-10
- acids-bases-and-salts
- acids-bases-and-salts-class-10-pdf
- acids-bases-and-salts-class-7
- difference-between-acids-bases-and-salts
- bbc-bitesize-acids-bases-and-salts
- acids-bases-and-salts-case-based-questions
- acids-bases-and-salts-class-10-notes-byjus
- salts-acids-and-bases
- acids-bases-and-salts-solutions
- chemistry-acids-bases-and-salts
- science-acids-bases-and-salts
- acids-bases-and-salts-class-10-notes
- acids-salts-and-bases
- acids-bases-and-salts-notes-pdf-download
- acids-bases-and-salts-definition
- define-acids-bases-and-salts
- how-do-acids-bases-and-salts-compare
- acids-bases-and-salts-pdf
- ncert-exemplar-acids-bases-and-salts-class-10
- edexcel-igcse-chemistry-acids-bases-and-salts
- acids-bases-and-salts-class-7-extra-questions
- acids-bases-and-salts-class-10-exemplar
- acids-bases-salts-pdf
- how-do-acids-and-bases-combine-to-form-salts
- mcq-from-acids-bases-and-salts-grade-10
- notes-for-acids-bases-and-salts-class-10
- mcq-for-acids-bases-and-salts-class-10
- g-class-10-notes-on-acids-bases-and-salts
- acids-bases-and-salts-grade-7
- grade-10-acids-bases-and-salts-pdf
- how-do-acids-bases-and-salts-differ
- how-to-tell-if-salts-are-acids-or-bases
- class-10-acids-bases-and-salts-notes
- class-10-acids-bases-and-salts-pdf
- class-10-acids-bases-and-salts
- ch-acids-bases-and-salts-class-10
- jamb-questions-on-acids-bases-and-salts
- prashant-kirad-acids-bases-and-salts
- acids-bases-and-salts-class-10-prashant-kirad
- acid-bases-and-salts-class
- learn-cbse-class-10-acids-bases-and-salts
- acids-bases-and-salts-class-10-learnohub
- acids-bases-and-salts-class-7-learn-cbse
- acids-bases-and-salts-used-in-our-daily-life
- 10th-cbse-acids-bases-and-salts-mcq
- class-7-acids-bases-and-salts-mcq
- acids-bases-and-salts-class-7-mcq-online-test
- mcq-of-acids-bases-and-salts-class-10
- m-class-10-notes-on-acids-bases-and-salts
- acids-bases-and-salts-class-10-ncert-pdf
- acids-bases-and-salts-notes
- acids-bases-and-salts-class-10-notes-pdf
- acids-bases-n-salts
- notes-for-acid-bases-and-salts
- questions-on-acids-bases-and-salts-class-10
- question-on-acids-bases-and-salts
- acids-bases-and-salts-class-10-ppt
- acids-bases-and-salts-questions
- acids-bases-and-salts-quiz
- acids-bases-salts-questions
- acids-bases-and-salts-questions-pdf
- acids-bases-and-salts-important-questions
- acids-bases-and-salts-reactions
- assertion-reason-on-acids-bases-and-salts
- acids-bases-and-salts-assertion-and-reasoning
- acids-bases-and-salts-class-10-all-reactions
- acids-bases-and-salts-class-10-give-reasons
- acids-bases-and-salts-class-10-solutions
- class-10-science-ch-acids-bases-and-salts
- class-7-science-acids-bases-and-salts
- class-10-science-acids-bases-and-salts-notes
- acid-bases-and-salt
- acids-bases-and-salts-class
- acids-bases-and-salts-class-10-notes-teachoo
- acids-bases-and-salts-class-10-textbook
- uses-of-acids-bases-and-salts
- acids-and-bases-salts
- acids-bases-and-salts-class-10-vedantu
- acids-bases-and-salts-class-10-video
- acids-bases-and-salts-class-7-vedantu
- acids-bases-and-salts-class-7-videos
- acids-bases-and-salts-worksheet
- acids-bases-and-salts-class-7-worksheet
- what-are-acids-bases-and-salts
- acids-bases-and-salts-class-10-worksheet
- class-x-acids-bases-and-salts-pdf
- class-x-acids-bases-and-salts
- acid-bases-and-salts-class-x-pdf
- acids-bases-and-salts-previous-year-questions
- science-ch-2-acids-bases-and-salts
- chemistry-acid-bases-salts
- acid-bases-and-salts-solution
- salt-base-or-acid
- acids-and-bases-and-salts-class-10
- chapter-1-:-acids-bases-and-salts-summary
- acid-bases-and-salts
- 2.-compare-and-contrast-acids-bases-and-salts
- chapter-2-acids-bases-and-salts-pdf
- chapter-2-acids-bases-and-salts-notes
- ch-2-acids-bases-and-salts
- class-10-ch-2-acids-bases-and-salts-notes
- acids-bases-and-salts-3-marks-questions
- chapter-4-acids-bases-and-salts
- chapter-4-acids-bases-and-salts-class-7
- class-7-science-ch-4-acids-bases-and-salts
- acids-bases-and-salts-form-4
- chapter-5-acids-bases-and-salts
- acids-bases-and-salts-chart
- class-6-acids-bases-and-salts-pdf
- acids-bases-and-salts-class-6
- acids-bases-and-salts-class-7-notes
- acids-bases-and-salts-class-7-mcq
- acids-bases-and-salts-class-7-pdf
- acids-bases-and-salts-class-8
- acids-bases-salts-notes
- acids-bases-and-salts-class-9
- acid-bases-and-salts-class-9-notes
- acids,-bases-&-salts
- what-are-salts-acids-and-bases
- is-salt-a-base-or-acid
- #acids
- #bases
- #salts
- #acidsbases
- #acidsbasessalts